Adeno-associated virus (AAV), as an important gene delivery and expression tool, has been widely used in the fields of scientific research and gene therapy in recent years. As a gene delivery system, AAV viral vectors have the advantages of good safety, low immunogenicity, the ability to infect dividing cells and non-dividing cells, and the ability to mediate long-term and stable expression of genes. These properties make AAV an ideal gene vector. However, you may have some doubts or usage problems in the actual scientific research application process. Here is a summary and analysis of the main issues, hoping to provide you with some help.
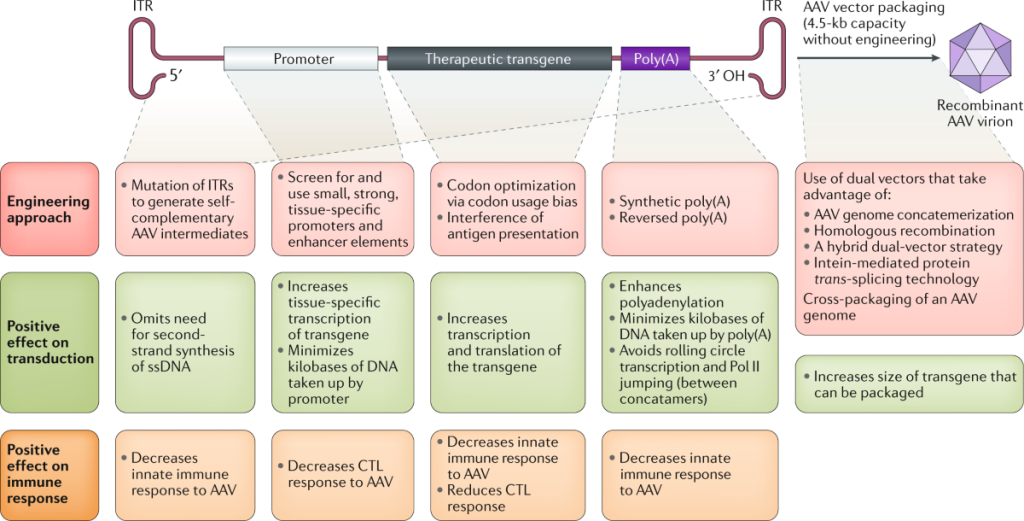
Figure 1. Engineering the AAV cassette.(Li C, et al.; 2020)
Q: Are there any biosafety issues when using AAV viruses?
A: To date, AAV has not been found to be pathogenic in humans. It is reported that 8 out of 10 people will be infected with AAV during their lifetime without causing related diseases. We generally use recombinant AAV (rAAV), which removes 96% of the wild-type AAV genome, further ensuring safety. rAAV generally does not have the ability to replicate and has a low host genome integration rate, small molecular weight, and extremely low immunogenicity, so it is highly safe. It is not only used in animal research, but also is a very safe viral vector even when used clinically. The clinical application of rAAV in gene therapy is a good example. In addition, NIH also pointed out that AAV belongs to the RG1 category of biological agents, which are low-risk biological agents that are not related to human and animal diseases. Therefore, just pay attention to routine safety protection and disinfection during AAV experimental operations.
Q: What do AAV serotypes mean?
A: AAV serotype refers to the protein shell that wraps genetic material, that is, the capsid protein, encoded by the Cap gene, which is the key for the virus to recognize and bind to cell surface receptors. The difference between different serotypes lies in the differences in the amino acid sequence and spatial structure of the capsid protein, which results in different serotypes having different tropisms for specific tissues or cells. AAV serotypes can be directly expressed as AAV2, AAV5, AAV9, etc. Another point that needs to be made clear is that the core skeleton of almost all recombinant AAV viral vectors is derived from the AAV2 subtype, so the complete AAV serotype can be expressed as, for example, AAV2 /2, AAV2/5, AAV2/9, etc.
Q: What are the roles of specific AAV serotypes and specific promoters?
A: AAV serotypes are based on the characteristics of virus infection, and the role of specific promoters is at the level of gene expression after virus infection. Generally speaking, specific promoters refer to tissue-specific promoters or organ-specific promoters. They are promoters derived from genes that are only expressed in certain specific tissues or organs, and the promoter activity is relatively good. With the blessing of its specific expression function, AAV with specific serotypes has stronger targeting ability. However, one thing that needs to be clear is that the regulation of cell expression in organisms is very complex. In actual application, not all specific promoters will strictly follow expression specificity. To a certain extent, it can be defined as relative specificity, so sometimes in animal experiments, it is possible to find that relevant genes are expressed in some non-target tissues, which is a promoter expression leakage incident. In this case, attempts can be made to further increase targeting specificity through in situ tissue injection.
Q: How long does it take to observe the effects after AAV injection into animals?
A: AAV is a single-stranded DNA virus. After infecting cells, it needs to form a double-stranded DNA form in the cell for gene expression. This process is generally long, and expression begins 1-2 weeks after the virus is injected, but the expression level at this time is relatively low. It is not suitable for sampling and testing, and it is usually recommended to conduct subsequent testing after 3-4 weeks. For AAV that wants to express quickly, you can choose scAAV, which is a self-complementary AAV whose DNA is double-stranded. Compared with standard single-stranded ssAAV, scAAV vectors do not require the step of replicating single-stranded DNA into double-stranded DNA to perform transcription, so gene expression It is more rapid and can reach the peak expression 3-5 days after injection, and the expression level is higher. However, their packaging capacity (<2.5Kb) is half that of ssAAV (<4.8Kb), limiting the genes that can be successfully packaged and the number of regulatory elements that the vector can have.
Q: How to improve infection efficiency when AAV is used to infect animals?
A: Excluding the influence of the status of the animal itself, from the perspective of AAV use alone, we need to select the appropriate AAV serotype, injection method, injection site, and injection volume based on the target of infection, and then by consulting references and virus usage instructions. Generally, AAV with a higher titer will be more effective than the same amount of AAV with a lower titer.
Q: Why is no fluorescence or weak fluorescence observed after AAV infects animals?
A: Here we need to consider the characteristics of the fluorescent protein, such as the brightness and stability of the fluorescent protein. The organic solvents used in the process of making tissue sections may change the structure of the fluorescent protein, causing fluorescence quenching; GFP is easily quenched in an acidic environment.
Q: Can fluorescence be directly observed on paraffin tissue sections to determine infection efficiency?
A: Can’t. Although paraffin sections can better preserve the complete tissue structure, the production process requires the use of organic solvents such as alcohol and xylene, which will cause the conformation of the fluorescent protein to change and prevent it from being excited to produce fluorescence. This situation can be observed by immunofluorescence staining of fluorescent proteins.
Q: When it is necessary to observe in vivo imaging of animals, is it better to carry a fluorescent protein gene or a luciferase gene in AAV?
A: In terms of comparison between fluorescent proteins and luciferase, luciferase is more suitable for in vivo imaging observation. It has stronger fluorescence penetration and is catalyzed by its substrate to emit light (i.e. bioluminescence), so the observed background is low and The effect is good, and the substrate has almost no impact on the physiological state of animals; fluorescent proteins require excitation light of a specific wavelength to excite fluorescence. Interestingly, animal hair and other tissues will also exhibit fluorescence, resulting in background that affects observation; By removing hair from the observation area, the background can be reduced.
Q: Can adeno-associated viruses (AAV) be used for genome editing?
A: The answer is yes. Using Crispr/Cas9 technology for in vivo knockout through AAV, a problem that needs to be solved is how to effectively load gene editing tools. At present, many Cas proteins with smaller genes have been discovered, such as SaCas9 (Staphylococcus aureus Cas9), Nme2Cas9 (Neisseria meningitides Cas9), etc. These Cas proteins can be effectively loaded into AAV vectors to achieve targeted gene editing in vivo. Another method is to use the cre-loxp system for knockout. The premise is that flox animals must be available, and then used with AAV-cre to achieve target gene knockout.
Q: Animals need to be modeled. When is the appropriate time to inject AAV?
A: The peak expression of AAV is 3-4 weeks after injection. The injection timing is then calculated based on the time of animal modeling and the purpose of injecting AAV. For example, if AAV regulates gene expression and thus plays a therapeutic role, the peak expression period of AAV must be controlled in synchronization with the modeling process; if it is for prevention, then it may be necessary to keep AAV at the peak expression period before or early in the modeling process. Of course, specific issues still need to be analyzed in detail and judged based on the characteristics of the model. It should be noted that there is no specific time frame for how long the peak expression of AAV can be maintained; if the modeling time is long, a follow-up injection can be performed 1-2 months after the first injection to maintain a long-term high expression state.
Q: Can AAV be used to infect cells in vitro?
A: In general, it is not recommended to use AAV to directly infect cells in vitro. Although AAV can maintain long-term gene transcription expression, in vitro cell lines usually proliferate quickly, causing the AAV infected into the cells to be continuously diluted as the cells divide, further reducing its expression level and thus limiting the expression ability of AAV. If it must be used for in vitro cell infection, it is best to use specific serotypes (such as AAV-DJ type), and the infection process requires a very high MOI (104~106). The specifics need to be explored in preliminary experiments.
| Cat. | Product Name | Application | Sample | |
| DEIASL347 | Human Anti-AAV2 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL343 | Human Anti-AAV5 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL344 | Human Anti-AAV6 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL345 | Human Anti-AAV8 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL348 | Human Anti-AAV9 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL345M | Mouse Anti-AAV8 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL345MQ | Mouse Anti-AAV8 Antibody ELISA Kit (Quantitative) | Quantitative | Serum | Inquiry |
| DEIASL348M | Mouse Anti-AAV9 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL347Y | Monkey Anti-AAV2 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL343Y | Monkey Anti-AAV5 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL344Y | Monkey Anti-AAV6 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL345Y | Monkey Anti-AAV8 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIASL348Y | Monkey Anti-AAV9 Antibody ELISA Kit | Qualitative | Serum | Inquiry |
| DEIA589 | AAV2 Titration ELISA Kit | Quantitative | Cell culture supernatants and purified virus preparations | Inquiry |
| DEIA591 | AAV5 Titration ELISA Kit | Quantitative | Cell culture supernatants and purified virus preparations | Inquiry |
| DEIAAV6 | AAV6 Titration ELISA Kit | Quantitative | Cell culture supernatants and purified virus preparations | Inquiry |
| DEIAAV8 | AAV8 Titration ELISA Kit | Quantitative | Cell culture supernatants and purified virus preparations | Inquiry |
| DEIAAV9 | AAV9 Titration ELISA Kit | Quantitative | Cell culture supernatants and purified virus preparations | Inquiry |