In chemistry, biochemistry, and pharmacology, the dissociation constant (KD) is a specific type of equilibrium constant used to measure the tendency of larger objects to reversibly separate (dissociate) into smaller components, such as When the complex decomposes into its component molecules or when the salt decomposes into its component ions. The dissociation constant is the reciprocal of the association constant. In the special case of salt, the dissociation constant can also be called the ionization constant. Generally expressed as:
AxBy = xA+ yB
The complex AxBy is decomposed into x A subunits and y B subunits, and the final dissociation constant can be expressed as:
KD=[A]x[B]y/[AxBy]
Where [A], [B] and [AxBy] are the equilibrium concentrations of A, B and the complex AxBy, respectively.
In the process of binding a biological antibody to an antigen, KD is the equilibrium dissociation constant between the antibody and its antigen, which is the calculated ratio of Koff/Kon. The association constant (Kon) is used to characterize the rate at which an antibody binds to its target. The dissociation constant (Koff) is used to measure the rate at which the antibody dissociates from its target. KD is inversely proportional to affinity, so the lower the KD value (the lower the concentration), the higher the affinity of the antibody. High-affinity interactions are characterized by low KD, rapid recognition (high Kon), and strong stability of the formed complex (low Koff). Therefore, KD can be used to evaluate antibody affinity or sensitivity. For example, when the KD value is 10-4 to 10-6, the antibody sensitivity is micromolar; when the KD value is 10-7 to 10-9, the antibody sensitivity is nanomolar; When the KD value is 10-10 to10 -12, its antibody sensitivity is picomolar concentration; when KD value is 10 -13 to 10 -15, its antibody sensitivity is femtomolar concentration.
H4ow to measure KD value?
Antibody binding affinity is an important parameter that can be used for antibody verification, which refers to the strength of the binding of antibody molecules to epitopes. KD and affinity are inversely proportional, so the antibody affinity can be calculated by detecting KD.
The affinity determination of monoclonal antibodies can perform high-precision detection because they are only selective for the same epitope; but in the case of polyclonal antibodies, because they detect epitopes that are heterogeneous and have different affinities Antibody mixture. Therefore, only the average affinity can be obtained. In order to determine antibody affinity, the following methods exist. These methods include ELISA-based methods, as well as other biophysical methods, such as micro-scale thermophoresis (MST) and surface plasmon resonance (SPR).
ELISA-Based Method
ELISA is the most popular method for studying antibody affinity. They do not require the use of large amounts of antibodies and antigens, nor do they require purification of proteins. In this method, a fixed concentration of antibody is incubated with the antigen in the solution until stable. Then, the concentration of unbound antibody was measured by indirect ELISA. This method requires a preliminary experiment to determine the antibody concentration used in the linear range of the ELISA reaction.
Micro Thermophoresis (MST)
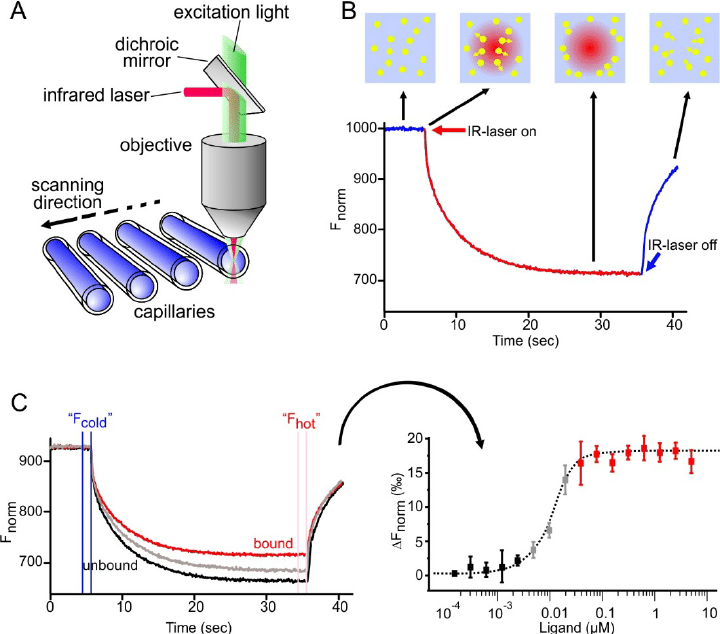
MST is a biophysical method that can detect the affinity of antibody molecules at various concentrations. It judges the affinity between molecules by measuring the movement of molecules along the microscopic temperature gradient induced by infrared lasers. This movement depends on many factors, including the hydration shell, charge, and molecular size. These molecules are initially uniformly distributed in the solution and can diffuse freely. When the infrared laser is turned on, unbound molecules usually move out of the heating spot. The binding of one molecule to another molecule (such as antibodies and antigens) causes changes in the overall temperature gradient. Then fluorescent labeling can be performed after the molecules move to obtain the affinity parameters of the antibody molecules.
Surface Plasmon Resonance (SPR)
SPR is an optical technology used to detect molecular interactions, in which one molecule is fixed in a metal film (ie a sensor chip) and the other molecule is movable. The combination of these molecules changes the refractive index of the film. Therefore, when polarized light is irradiated on the film, the extinction angle of the light changes and can be monitored by an optical detector.
In antibody affinity measurement, the antibody to be analyzed is first captured by a high-affinity anti-IgG antibody immobilized on the surface of the sensor chip. Then a series of concentrations of antigen are sequentially injected across the surface. According to the sensor graph curve, one can rank antibodies according to kinetic parameters and screen the monoclonal antibodies with the best characteristics. First, the universal anti-antibody (such as rabbit anti-mouse immunoglobulin) is covalently attached to the surface of the chip, and then the first antibody is captured by the previously attached universal antibody. After blocking the unsaturation site, the injected antigen will be bound by the first antibody to form an Ab-Ag complex. After administration of the second monoclonal antibody (used to assess binding affinity), if the second antibody binds to a different independent epitope, a unique sensorgram curve will be observed.
