Snake bites seriously threaten public health security in tropical and subtropical countries and regions. Due to the high mortality and disability rates caused by venomous snake bites, there are an estimated 1.8 to 2.7 million snake bites worldwide each year, with 81,000 to 138,000 related deaths. Snake venom protein components and their abundance are closely related to the symptoms caused by snake bites. Rapid identification of the type of venomous snake bite is very important to obtain the best clinical treatment as early as possible. Snake venom is a natural protein secreted by the venom glands of venomous snakes. Its chemical composition is complex and diverse. The main toxic components include peptides, metalloproteinases, thrombin, etc.
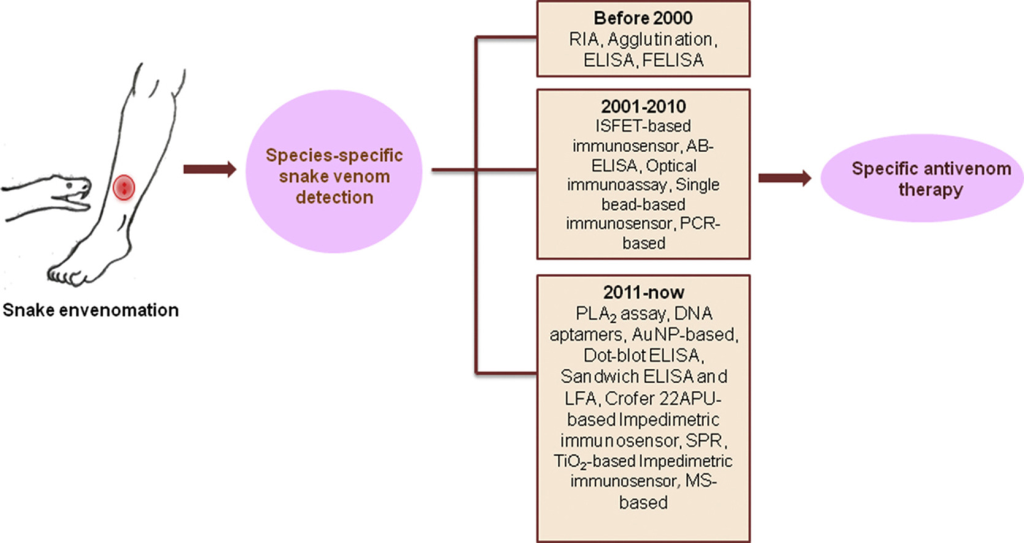
Figure 1. Recent developments in diagnostic tools and bioanalytical methods for analysis of snake venom.
According to statistics from the World Health Organization, disability and death from venomous snake bites are still common phenomena in developing countries. Before the use of antivenom, there is still a general lack of feasible experimental methods for snake venom detection. At present, the clinical identification of snake bites mainly relies on the patient’s description of the snake’s morphology, the manifestations of the bite site and systemic symptoms, and simple tests such as coagulation, blood routine, and urine routine. However, the appearance of some non-venomous snakes is similar to that of venomous snakes. Clinically, it is also encountered that venomous snakes “dry bite” without detoxifying without poisoning. In addition, the limitations of these diagnostic methods include errors in patient description, or the time it takes for symptoms to appear after a venomous snake bite, and the lack of specificity in blood biochemistry and urine tests. Snake venom is a highly complex mixture. There are still certain differences in the composition and toxicological effects of snake venom between snakes of the same family, the same genus, or even different families and genera, or a certain snake species in different regions. Therefore, identification of the species of the injuring snake is crucial to the effectiveness of early use of monovalent antivenom in the treatment of venomous snake bites. For a long time, researchers have been committed to developing a stable, reliable, fast, simple, and specific snake venom identification method. Currently, there are radioimmunoassay, agglutination assay, enzyme-linked immunosorbent assay (ELISA), fluorescent immunoassay, and proteomics techniques have been used to detect various snake venoms and toxins.
Radioimmunoassay (RIA)
RIA uses radioactive isotopes (such as 3H, 125I, 131I, etc.) to label antigens or labeled antibodies to determine the corresponding antigen, and uses special instruments to monitor the metabolism of the labeled antigen or labeled antigen-antibody complex and calculate the number of antigens to be tested. Compared with double-antibody sandwich ELISA, this method has simpler operation steps and requires less body fluid. However, this method uses radioactive materials and is expensive. In addition to problems related to the short half-life of 125I, it also requires specialized and sophisticated reading equipment to measure isotope levels. It has proven impractical and not very operable in clinical patients. Compared with double-antibody sandwich ELISA, this method has simpler operation steps and requires less body fluid. However, this method uses radioactive materials and is expensive. In addition to problems related to the short half-life of 125I, it also requires specialized and sophisticated reading equipment to measure isotope levels. It has proven impractical and not very operable in clinical patients.
Enzyme-linked Immunosorbent Assay
ELISA is an immunological diagnostic technology that uses enzyme-labeled antigens or antibodies to bind to the corresponding antibodies or antigens in the sample to be tested. The basic principle is to physically adsorb the antigen or antibody to the surface of a certain solid phase carrier while maintaining immune activity, and the protease and the corresponding antibody or antigen are coupled to form an enzyme-labeled antibody or antigen, and the enzyme-labeled antibody or antigen is simultaneously It has immunological activity and enzymatic activity. After combining with the antigen or antibody on the surface of the solid-phase carrier, the enzyme is used as the detection signal. After the enzyme reaction substrate is added, it is catalyzed by the enzyme to produce a colored product. The amount of the product is equal to the amount of the antigen or antibody to be tested. Proportional, according to the color depth, it can be qualitative or a special microplate reader can be used to detect the adsorption signal for quantitative analysis. Use biotin or avidin to amplify the signal to further increase the sensitivity of the experiment. Due to its advantages of sensitivity, specificity and rapidity, ELISA is suitable for testing large quantities of samples and is an internationally recognized standardized diagnostic method.
Liquid Chromatography-Mass Spectrometry (LC-MS)
LC-MS can effectively separate and analyze complex organic mixtures. Liquid chromatography has strong separation capabilities and can effectively separate thermally unstable and high-boiling compounds in mixed organic matter. Combined with the powerful component identification capabilities of mass spectrometers, Analyze the isolated organic compounds one by one to identify the molecular weight, structure and concentration of the organic compounds. Due to the powerful electrospray ionization technology of LC-MS, its mass spectrum is simple and intuitive, and subsequent data processing is simple. Therefore, LC-MS is an essential tool for analyzing organic compounds. Snake venom is a mixture of proteins, enzymes, small amounts of metal ions, carbohydrates and amines with different molecular weights. Its composition is complex and has a variety of biological activities and pharmacological effects. Proteomics technology can be used to study the evolution of venom proteins in different snake genera or the same snake genus in different regions, the relationship between protein structure and function, and the classification status of venomous snakes. With the maturity of the development of proteomics technology, snake venom proteomics has been deeply studied, revealing the components of different snake venom proteins.
Polymerase Chain Reaction (PCR)
PCR is a molecular biology technology used to amplify and amplify target DNA or RNA fragments. The target DNA or RNA is replicated in vitro. The biggest feature of PCR is that it can greatly increase trace amounts of gene fragments.
Fluorescent Immunoassay (FIA)
FIA is a non-radioactive labeling immunoassay technology based on enzyme-labeled immunoreactants and highly active enzyme-catalyzed substrate color or luminescence methods to achieve the purpose of quantitative analysis. Fluorescence methods were introduced into immunological analysis to improve the sensitivity of immunoassays. 4-Methylumbelliferyl phosphate is widely used as a fluorescent substrate in combination with alkaline phosphates.
Immunosensor
Immune sensors are biosensors, also known as optical immune sensors. Their key information converters use photosensitive elements and work using optical principles. Biorecognition molecules (protein antigens, etc.) are solidified on the sensor and interact with light in the optical element, causing the optical signal to change. The immune response is detected by detecting the optical signal caused by the physical change in the thickness of the molecular film on the optical silicon chip. Therefore, the test object can be analyzed and measured.
Related Products