The early stages of apoptosis signal transduction involve a complex cascade of signal molecules. Among them, early apoptosis signal transduction focuses on the activation of signal transduction molecules downstream of death receptors and/or pro-apoptotic members of the B-cell lymphoma 2 (Bcl-2) pathway. By detecting the early events of apoptosis, the pathway (external or internal) that induces apoptosis can be determined. Although there are no obvious signs of apoptosis in the early stages, cell signal transduction events include post-translational modification (PTM) and the assembly of signal transduction complexes. Therefore, traditional Western blotting is usually the best choice for studying the proteomic changes in the early stages of apoptosis.
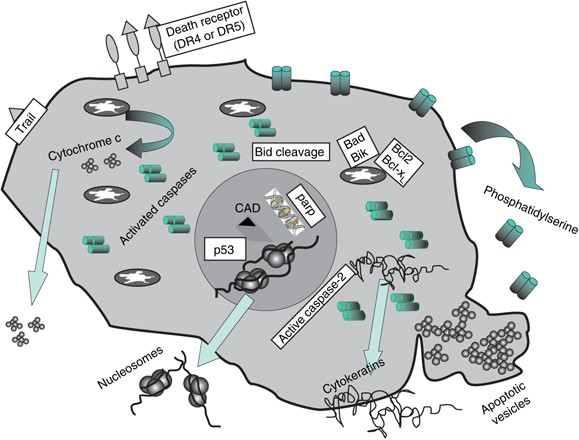
In the process of apoptosis, caspases (cysteine-aspartic proteases) promote cell death through the hydrolysis of more than 400 proteins. Studies have found that caspase is mainly activated through internal and external cell death pathways.
The intrinsic cell death pathway is a pathway that is mainly regulated by Bcl-2 family proteins and completes cell death through mitochondria. The key step in the intrinsic cell death pathway is the permeabilization of the outer mitochondrial membrane. After the outer mitochondrial membrane is permeabilized, the release of proteins from the space between the mitochondrial membranes promotes the activation of caspase and cell apoptosis. The released cytochrome C binds to APAF-1 and induces caspase-9 activation. Caspase 9 then activates caspase 3 and 7, leading to apoptosis.
Early Markers of Intrinsic Apoptotic Pathway
Bcl-2 Family
The Bcl-2 family regulates cell apoptosis through the effects of pro-apoptotic and anti-apoptotic members. This family consists of more than 20 cytoplasmic proteins containing Bcl-2 homology (BH) domains, which are essential for the function of apoptosis. Each member of the Bcl-2 family contains at least one BH motif to promote its function. The members of the Bcl-2 family are divided into three functional categories, namely anti-apoptotic, pro-apoptotic multi-domain effector and only Bcl-2 homolog 3 (BH3) activator.
Among them, the main family members are:
Bcl-2
Bcl-2 is the initial member of the Bcl-2 family and plays a role in inhibiting cell apoptosis. Bcl-2 is a ubiquitously expressed mitochondrial membrane protein, which can inhibit apoptotic cell death by isolating BAX and BAK.
PUMA
PUMA, also known as Bcl-2 binding component 3 or JFY-1, is a member of the BH3-only protein family. Studies have found that PUMA expression is regulated by p53 tumor suppressor protein. When cells respond to stress and DNA damage, the up-regulated p53 binds to the PUMA promoter, resulting in PUMA transcription. PUMA acts as a triggering factor for apoptosis through mitochondrial signal transduction and interaction with Bcl-xL, Bcl-2 and other anti-apoptotic proteins.
NOXA
NOXA is the pro-apoptotic member of BH3-only in the Bcl-2 protein family. Studies have found that NOXA is located in mitochondria and is the transcription target of p73 and p53. NOXA replaces the pro-apoptotic proteins BAK and BIM from members of the anti-apoptotic protein Bcl-2 family, thereby promoting apoptosis during cell survival. In addition, NOXA can also be upregulated independently of p53.
BAD
BAD is the only pro-apoptotic member of BH3 in the Bcl-2 protein family. BAD has a pro-apoptotic effect, and its exact role in apoptotic signal transduction depends on the post-translational modification status of BAD. Non-phosphorylated BAD forms heterodimers with two anti-apoptotic Bcl-2 protein family members, Bcl-xL and Bcl-2. The combination of BAD and Bcl-xL promotes cell apoptosis by inhibiting the anti-apoptotic function of Bcl-xL. After phosphorylation of serine, BAD cannot heterodimerize with Bcl-xL. This combination retains BAD in the cytoplasm, allowing Bcl-xL to inhibit cell apoptosis.
BID
BID is a pro-apoptotic molecule that can form heterodimers with other members of the Bcl-2 family (including the agonist BAX or the antagonist Bcl-2). BID contains a BH3 domain, which is required for its interaction with Bcl-2 family proteins and its pro-apoptotic activity. BID is cleaved to produce a shorter active form called truncated BID (tBID). tBID translocates to the mitochondria, leading to the activation of the intrinsic pathway.
BAX
BAX, also known as Bcl-2-like protein 4 or Bcl-2-related X protein, is a member of the pro-apoptotic multi-domain effector family. After inducing apoptosis, BAX translocates to mitochondria and induces MOMP.
BAK
BAK is a member of the pro-apoptotic multi-domain effector family. Due to its similar structure to BAX, it is considered to have significant homology with BAX. The study found that Mcl-1 and Bcl-xL isolate BAK in non-apoptotic cells. After the onset of apoptosis, NOXA and BAD release BAK, which causes the outer mitochondrial membrane to permeate and release pro-apoptotic factors.
Extrinsic Cell Death Pathway
After the death receptor binds to the ligand, the receptor oligomerizes and recruits adaptor proteins, such as serine/threonine protein kinase 1 (RIPK1), RIPK3, and Fas-related proteins ( FADD), eventually formed as a death-inducing signaling complex (DISC). As part of the DISC assembly, the former caspase 8 can be cleaved into its active form caspase-8 by oligomerization, which in turn cleaves and activates the effector caspase (caspase 3 and caspase 7). One of the early effects of this activation is the inhibition of phosphatidylserine (PS) flippase, resulting in exposure of PS on the outer plasma membrane.
Early Markers of Extrinsic Apoptotic Pathway
Phospholipid Asymmetry
Phospholipid asymmetry is the controlled distribution of different lipid species in the lipid bilayer. One of the early apoptotic events is the rearrangement of lipids in the plasma membrane. This change causes PS to be exposed on the outer surface of the cell. This exposure depends on the inhibition of flippase and the signal from the phagocytic cell.
Annexin V
Fluorophore-conjugated annexin V is commonly used to assess changes in plasma membrane asymmetry. However, PS exposure may be short-lived, and this phenomenon is called “PS flip”. In the early detection of apoptosis, Annexin V and pSIVA can be used together with propidium iodide to distinguish dead cells from cells in the early stages of apoptosis.
Initiator Caspase
Caspase-8 is the promoter caspase, a key signal molecule in the apoptosis mediated by CD95 and tumor necrosis factor receptor 1 (TNFR1). After activation, the cell death receptor Fas is connected to the adaptor molecule FADD through the corresponding death domain (DD). Then, FADD binds to procaspase 8 through the death effector domain (DED), and then undergoes oligomerization and autocatalytic activation. Activated caspase-8 cleaves and activates effector caspase and Bcl-2 family member BID. The activity of the starting protein caspase (caspase-8 and caspase-10) can be determined by Western blot or flow cytometry. The determination of caspase-8 activity by Western blotting depends on the detection of caspase-8 active fragments with a suitable caspase antibody.
Effector Caspases
Caspase-3 and caspase-7 are called “effector” caspases. The promoter caspase is automatically proteolyzed, and the effector caspase is cleaved by the promoter caspase. This hierarchical structure allows amplification of chain reactions. Effector caspases control many of the phenotypic changes observed during apoptosis, such as membrane blistering and DNA fragmentation.
