Since the discovery of cowpox inoculation to prevent smallpox in the 18th century, vaccines have become an important means for human beings to resist pathogen infection and prevent the occurrence of diseases. Although immunization has eliminated or controlled the spread and pathogenic hazards of some important pathogens in humans, many widespread infectious diseases worldwide, especially those with viruses as pathogens, such as human immunodeficiency virus (HIV), influenza Viruses, highly pathogenic coronaviruses, etc. still pose great challenges to the research and development of vaccines. In recent years, with the continuous development and progress of theories and technologies such as immunology theory, biological genetic engineering, high-throughput sequencing and big data analysis, a series of new technologies and new platforms for vaccine research and development have provided more feasible options for the research and development of virus vaccines. This paper briefly introduces the research and development status of virus vaccines in the way of classification of general virus vaccine types.
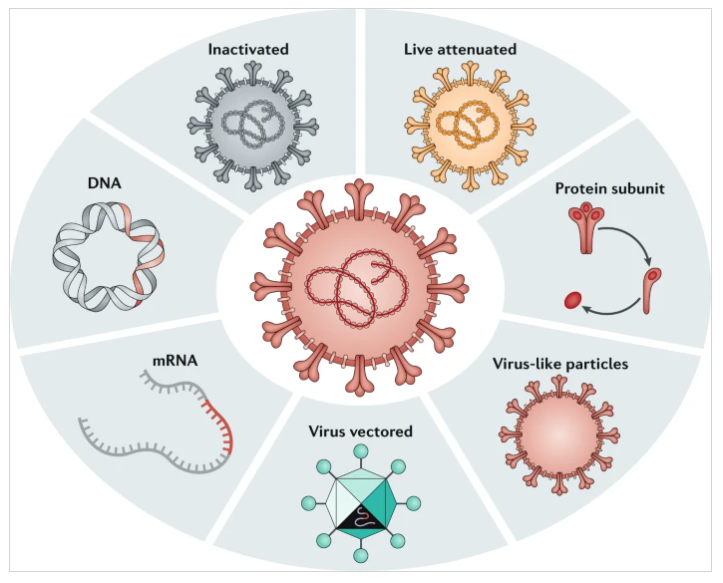
Inactivated Virus Vaccine
Inactivated virus vaccine is a vaccine made by physically or chemically killing the virus, and it is the most mature virus vaccine development technology. At present, inactivated hepatitis A vaccine, inactivated hand, foot and mouth disease vaccine, inactivated polio vaccine and other products have been successfully developed and widely used for many years. The advantages of inactivated vaccines are that the technology is mature, can be rapidly developed using existing production conditions and technologies, and has good stability. Different from live attenuated vaccines, inactivated virus vaccines are safer because they can deliver a range of different viral antigens and induce epitope conformation-dependent antibody responses; but they also have certain limitations. They are less immunogenic, often require adjuvants and require repeated injections. In addition, the cellular immune response induced by virus-inactivated vaccines is weak, and there may be a risk of antibody-dependent enhancement (ADE).
Live Attenuated Vaccine
Live attenuated vaccine is a vaccine made by treating the virus in a certain way to obtain a strain with reduced toxicity. The currently widely used measles vaccine is the live attenuated vaccine, which can effectively prevent the epidemic of measles. Live attenuated vaccines are usually passaged in cell culture to delete or mutate the virulence gene of the virus to reduce its pathogenicity. These deletion mutations make live attenuated vaccines poorly replicable in host cells but not disease-causing.
Subunit Vaccine
Subunit vaccines are produced by genetic engineering of the subunit proteins in the virus that are most likely to serve as antigens. At present, hepatitis B vaccine, cervical cancer vaccine and other products have been successfully developed and widely used. Subunit vaccines play a protective role mainly by inducing CD4+Th cells to produce antibody responses. Its advantages are that the ingredients are determined, the biosafety is high, the development process is mature, and it can be produced on a large scale. Similar to inactivated virus vaccines, the protein or polypeptide components of subunit vaccines are less immunogenic, not only require adjuvants, but also often require multiple injections, and their effect on activating CD8+ T cell immunity is weak. If an alum adjuvant was used, the immune response would be more Th2-biased, making it unsuitable for respiratory mucosal vaccination and possibly increasing the risk of ADE. To this end, subunit vaccines can be designed to focus on neutralizing epitopes, thereby avoiding the production of non-neutralizing antibodies to reduce the occurrence of ADE.
Virus-Like Particle Vaccine
Virus-like particles are particles that spontaneously assemble and form from multiple co-expressed or mixed viral proteins. These virus-like particles are highly structurally similar to viruses, but lack the viral genome and thus are not infectious. Some of these virus-like particle vaccines are produced in vivo by viral vectors expressing protein components, and many are produced in bacteria, yeast, and cell lines. A variety of hepatitis B vaccines on the market are virus-like particle vaccines, the principle of which is to produce hepatitis B virus surface antigen (HBsAg) through yeast or cell line expression systems and integrate them into spontaneously assembled virus-like particles.
Recombinant Viral Vector Vaccine
Recombinant viral vector vaccines use a genetically engineered virus as a vector. After the virus infects the human body, it expresses the antigenic protein of the virus to produce immunogenicity. Among them, adenovirus vector is the most commonly used. As an important vaccine development platform, recombinant viral vectors have good genetic plasticity and safety, and can induce strong cellular immune responses without adjuvants. Vaccines developed using some viral vectors, such as Ad5 and ChAd, can produce good immune protection after only one vaccination, and can be delivered to the respiratory mucosa for vaccination, thereby effectively resisting the invasion of respiratory viruses. In addition, viral vector vaccines also have the advantages of a short preparation period, rapid scale-up of the production process, and the ability to express high amounts of proteins.
Nucleic Acid Vaccine
Nucleic acid vaccines include recombinant plasmid DNA vaccines and mRNA vaccines. Recombinant plasmid DNA vaccines have been used as a mature vaccine research and development platform for many years, while mRNA vaccines have become a hot spot in the field of virus vaccine research and development as an emerging platform. The mechanism of action of mRNA vaccines is that mRNA encoding viral antigens can effectively enter the host cell cytoplasm through carriers such as lipid nanoparticles for protein translation and post-translational modification. Therefore, mRNA vaccines are synthesized through intracellular transcription, have no viral components, and are not infectious. These characteristics make them superior to other vaccines in terms of safety, efficacy and overcoming pre-existing immunity. mRNA vaccines have the advantage of rapid development. The same platform can develop vaccines against different pathogens and can respond quickly to outbreaks of infectious diseases.
