In recent years, the overall incidence of global malignant tumors has shown a continuous upward trend. At present, the main clinical treatment of malignant tumors is surgery, chemotherapy and radiotherapy, but it is difficult to achieve satisfactory results. In recent years, anti-tumor targeted drugs have made breakthroughs in the treatment of a variety of malignant tumors, making them a new treatment method following surgery, chemotherapy, and radiotherapy. However, currently developed targeted drugs still have many shortcomings. Although trastuzumab, which was approved by the U.S. FDA in 2010, has been successful in Her2-positive breast cancer, its efficacy on gastric and esophageal tumors is not ideal. Similarly, epidermal growth factor receptor (EGFR) and mammalian rapamycin-like target protein (mTOR) inhibitors have also been proven ineffective in multiple phase III clinical trials of gastric and esophageal tumors. CLDN18.2 protein is a highly specific and stable target protein expressed on the surface of a variety of malignant tumor cells. Claudiximab, which specifically binds to it, has recently been confirmed by a number of clinical trials to have significant efficacy, small adverse reactions and a larger safe dose range.
Claudins (CLDNs) Family and the Structure and Biological Function of Cldn18.2 Protein
CLDN18.2 protein is a transmembrane protein, which belongs to Claudins (CLDNs) family members. Its N-terminal and C-terminal are located in the cell, and the entire protein is expressed on the cell membrane. It is an important structural component of cell tight junctions. Claudins have 4 transmembrane regions, 2 extracellular loops and 1 intracytoplasmic loop, which are involved in the formation of tight junctions between cells. The CLDN18 gene is located at 3q 22.3 of human chromosome 3. There are two options for the first exon of the gene, which can express CLDN18.1 and CLDN18.2 two different splicing mutants, resulting in the inclusion of extracellular loop 1 in The internal amino-terminal 69 amino acid sequence difference, so the two extracellular epitopes are different. This amino acid difference can prevent the epitope of CLDN18.2 protein from immunologically cross-reacting with CLDN18.1 protein.
As a key protein in the tight junction structure between cells, the CLDNs family is widely distributed in various epithelial tissues. One type of epithelial cell can often express more than 27 CLDNs family members. The normal expression of these CLDNs proteins ensures that each tissue accurately regulates the tight connection function of specific tissues. Studies have found that CLDNs proteins are closely related to the maintenance of osmotic pressure, barrier function and cell polarity of epithelial cells, and are involved in the process of immune defense against pathogens. In addition, CLDNs have been confirmed to have changes in their expression patterns during the occurrence and development of many tumors. The study of targeted therapy using CLDNs lineages as specific marker proteins has received extensive attention. Although most CLDNs are widely expressed, individual members such as CLDN18 protein are often highly selectively expressed in specific tissues such as the gastrointestinal tract.
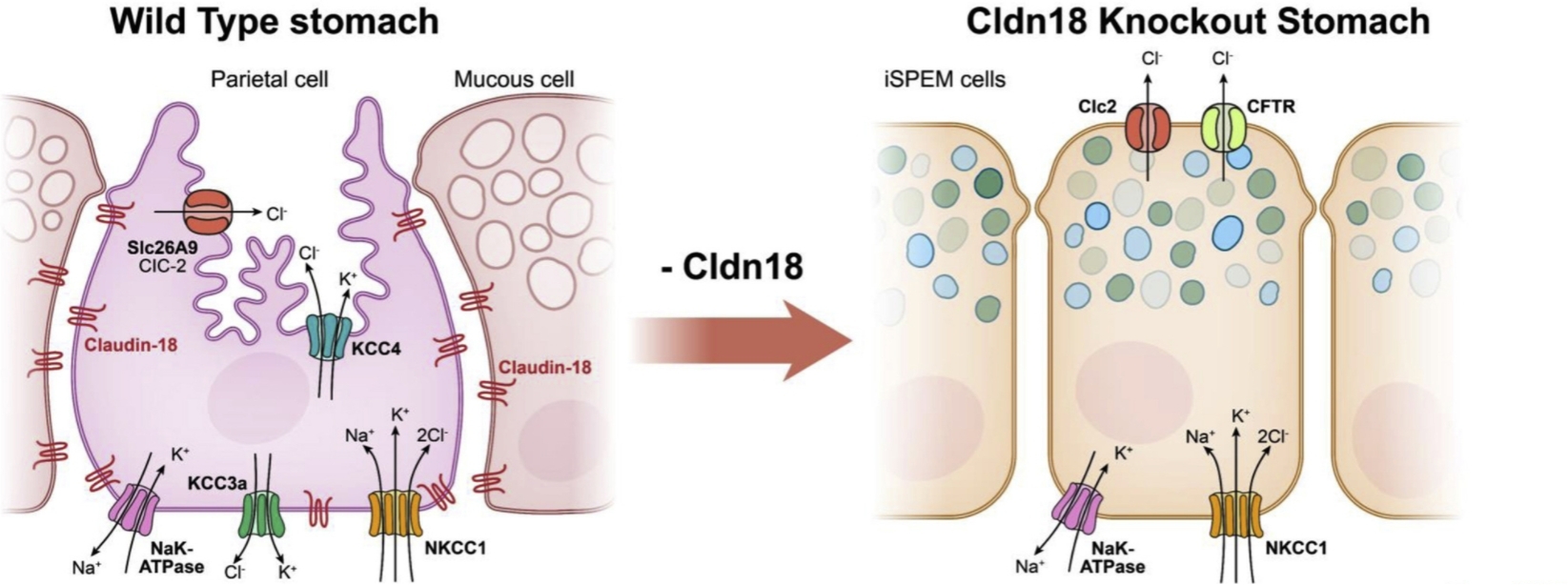
Among them, CLDN18.1 protein is a specific antigen selectively expressed by alveolar epithelial cells. It is only highly expressed in normal alveolar tissues, but not found in other normal tissues, including pancreatic ducts. CLDN18.2 protein is also a highly selective marker protein, but its distribution is completely different from CLDN18.1 protein. The expression of CLDN18.2 protein is highly restricted in normal healthy tissues. It is not expressed in undifferentiated gastric stem cells. It is only expressed in differentiated gastric mucosal membrane epithelial cells, and the expression level is very limited, which is conducive to maintaining the barrier function of gastric mucosa. It can prevent H+ in gastric acid from leaking through paracellular pathways. However, CLDN18.2 protein frequently undergoes abnormal changes during the development of a variety of malignant tumors. For example, when gastric epithelial tissue undergoes malignant transformation, the disorder of cell polarity will cause the CLDN18.2 protein epitope on the cell surface to be exposed. At the same time, CLDN18.2 gene will also be abnormally activated, highly selective and stably expressed in specific tumor tissues, and participate in the proliferation, differentiation and migration of tumor cells, which makes it an effective molecular target of potential anti-tumor drugs.
Expression of CLDN18.2 in Different Malignant Tumors
CLDN 18.2 protein is a CD20-like differentiated protein. Although its expression is highly restricted in normal tissues, it often has abnormally high expression during the occurrence and development of a variety of primary malignant tumors. CLDN18.2 protein was initially found to be consistently and stably highly expressed in a variety of gastric cancer tissues. However, subsequent studies have shown that it can also be abnormally activated and overexpressed in a variety of primary malignancies such as breast cancer, colon cancer, liver cancer, head and neck cancer, bronchial cancer, and non-small cell lung cancer, especially in malignant tumors of the digestive system, including gastric cancer (70%), pancreatic cancer (50%), esophageal cancer (30%), etc.
The expression of CLDN18.2 protein is not limited to the primary foci, it is also highly expressed in metastatic foci, and may be involved in the process of malignant tumor cell proliferation and chemotaxis. At present, the mechanism of CLDN18.2 protein promoting lymph node metastasis and distant metastasis of malignant tumors is not very clear, and its abnormal expression may be related to the structure and function of tight junctions between malignant tumor cells.
Regulation of CLDN18.2 Gene
The CLDN18.2 gene sequence is highly conserved in humans and many mammals, but its expression is prone to change during tumor progression, and its expression level increases significantly with the gradual infiltration of tumors. The regulatory mechanism of CLDN18.2 expression is still not fully understood. At present, it is believed that its expression is mainly affected by the hypomethylated gene sequence CpG island promoter in the coding sequence of CLDN18.2 gene and the transcription factor cyclic adenosine phosphate response element binding protein (CREB). The activation of CLDN18.2 depends on the binding of the transcription factor CREB to the hypomethylation site in the CpG island. The binding site of CREB is the highly conserved TGACGTG sequence, which is located between the CREB binding start site and the transcription start site Highly repetitive nucleotide sequence. After tumor cell cAMP activates protein kinase A (PKA), the activated PKA enters the nucleus and activates CREB through phosphorylation of the amino-terminal kinase inducible domain (KID). After phosphorylation by PKA, the transcriptional activity of CREB will increase by 10-20 Fold, combined with the CpG island promoter caused abnormal activation and overexpression of CLDN18.2. At the same time, the demethylation of DNA gene sequence has also been confirmed to significantly increase the expression of CLDN18.2, indicating that the regulation of CLDN18.2 is also related to DNA methylation.
