Apoptosis refers to the physiological process of autonomous and orderly death of cells controlled by genes in order to maintain a stable environment in the body.
Cell death in the body can occur through mechanisms including necrosis, mitotic disorders and autophagy. The morphological changes in the process of apoptosis mainly include the four processes of plasma membrane blistering, cell contraction, chromatin condensation and the formation of apoptotic bodies. The biochemical process can be divided into three stages: the activation of the initiating factor caspases, the release of apoptotic factors from the mitochondria, and the activation of the effector caspases, the latter lysing the known substrates and eventually disintegrating dead cells.
Significance of Apoptosis Markers
Clinical studies have shown that the inhibition of apoptosis is a hallmark of human cancer, and the ideal strategy for many targeted therapies is to specifically induce tumor cell death in vivo. Therefore, mechanism-based therapy in oncology can directly induce tumor cell apoptosis by targeting the molecular components of the apoptosis regulatory pathway, or indirectly induce apoptosis by regulating drug targets. Either way, there is an urgent need to apply proven and effective apoptosis biomarkers in clinical trials of apoptosis anticancer therapies. In addition, in basic apoptosis-related molecular mechanism research or tumor scientific research, it is also necessary to use accurate biomarkers to determine the relationship between research objects and apoptosis.
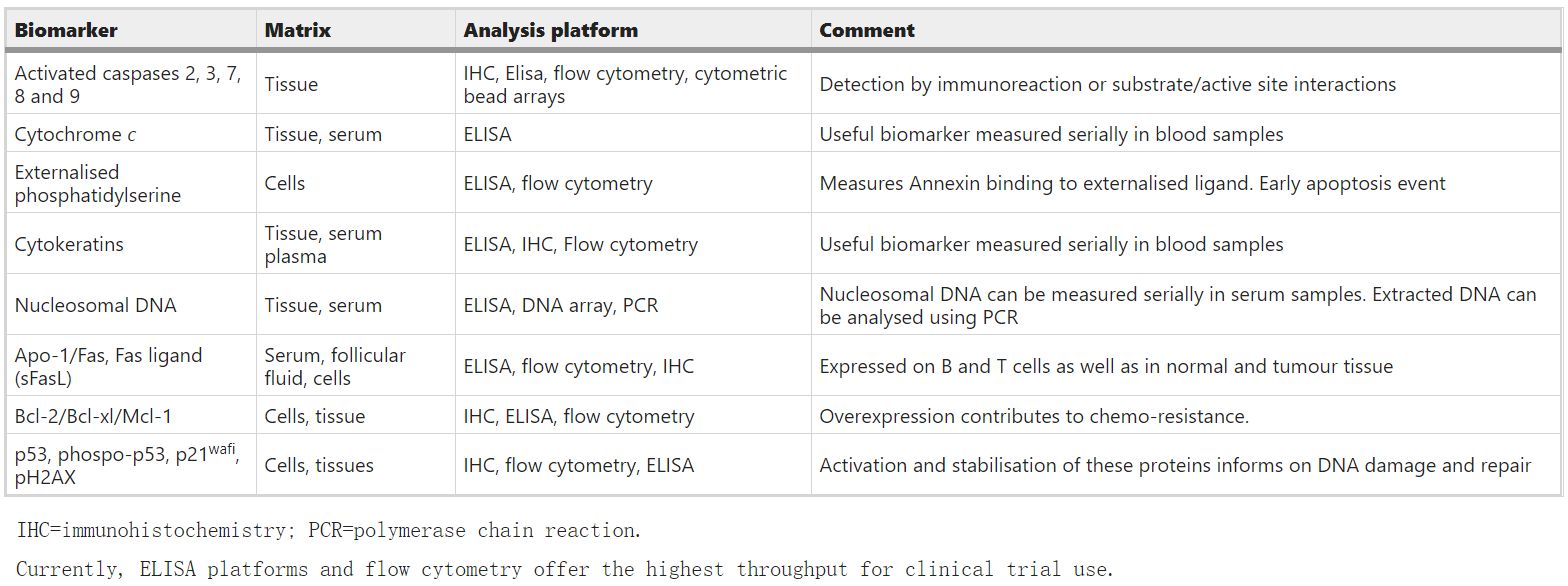
Molecularly, apoptosis is activated through death receptor-mediated external pathways or mitochondrial-directed internal pathways. The ligand binds to the death receptor on the plasma membrane, which triggers the activation of the initiator caspases 8 in an external pathway. In some cell types, this directly activates effector caspases, such as caspase 3. In other cells (and most cancer cells), caspase 8 can also amplify death signals by participating in internal pathways. The latter is controlled by pro-apoptotic and anti-apoptotic Bcl-2 family proteins. Under the stimulation of apoptosis, changes in the interaction of proteins within the family of mitochondria determine the release of cytochrome c. Cytoplasmic cytochrome c activates the apoptotic body complex, the promoter caspase 9 and the effector caspase. Afterwards, Caspase cleaves cytokeratins (CKs) and activates the activities of poly(ADP-ribose) polymerase and endonuclease (to generate nucleosomal DNA (nDNA)). These processes constitute a series of irreversible events and lead to the formation of apoptotic bodies. In addition, these processes promote the exposure of phosphatidylserine on the outer surface of the plasma membrane, allowing phagocytes to recognize dying cells.
Participants in many of the above molecular events are potential biomarkers of apoptosis (Fig 1). Usually in in vitro studies, most of these molecules can be used as markers to be detected by chemical methods. However, the detection of apoptosis in vivo is challenging. It is usually asynchronous, and the half-life of apoptotic cells in the tissue is very short. Therefore, biomarker analysis time is critical to the level of apoptosis detected in patient samples.
Ideal Characteristics of Biomarkers
Compared with in vitro studies, biomarkers used in clinical research have more stringent requirements. This marker must not only meet the requirements of scalable hypothesis testing in early clinical trials, but also confirm whether the drug has reached the tumor target (Proof of Mechanism, POM) and achieved the expected tumor results (Proof of Concept) in subsequent treatments , POC). PD biomarkers compared before and after drug treatment may reflect changes in the drug target (for example, protein phosphorylation, direct DNA damage, and enzyme activity), or those changes at the end of the target (for example, downstream signaling events, gene expression Variety). The cell fate after target regulation should be obvious (for example, changes in proliferation, apoptosis, or angiogenesis). The biomarkers measured in tumors and/or replacement body fluids cover a wide range of molecules (proteins, nucleic acids, lipids and sugars) as well as circulating intact cells. The ideal biomarker should provide a minimally invasive/non-invasive indirect continuous readout of disease/drug activity.
Therefore, ideal biomarkers should pursue the following standards:
- Specificity of biological process/target
- It can be accurately quantified in clinical samples and has sufficient dynamic range to detect changes after drug treatment
- Provide fast, reliable and powerful measurement
- It can be verified as an internationally recognized standard
- There is almost no overlap in levels between untreated patients and treated patients
- The baseline level does not vary from patient to patient
- Have a level related to the total burden of disease and not affected by unrelated conditions
- Have a level closely related to the proximal or distal effect of the treatment, thereby contributing to POM or POC
- Can be measured in easily available clinical samples
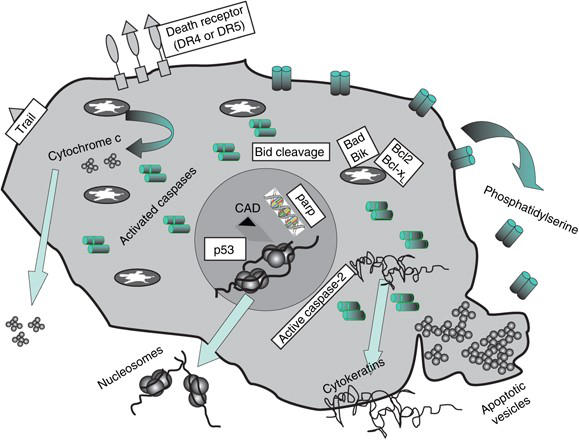
Common Apoptotic Markers
Table 1:Biomarkers of apoptosis.(Singhal et al.; 2005)
The exogenous apoptotic pathway and the intrinsic apoptotic pathway are the two main pathways leading to the execution of apoptosis, as well as the TNF pathway. The external apoptotic pathway that initiates apoptosis is triggered by death ligands that bind to death receptors present in the cell membrane. The intrinsic apoptotic pathway initiates apoptosis by activating Caspase-3 or cleaving the BH3 interaction domain death agonist (Bid) through the Puma and Noxa protein subgroups. The TNF pathway is mainly caused by the binding of TNF-α and TNFR1 through the intermediate membrane protein TNF receptor-associated death domain (TRADD) and Fas-associated death domain protein (FADD) to initiate the pathway leading to caspase activation. Therefore, apoptosis involves members of the Bcl-2 family (Bcl-2, Bcl-xL, Bcl-w, Mcl-1), Bax protein, caspase 8, death receptor, p53/p73 gene/P21 WAFI, and Changes in the level of NF-κB and other related genes can be detected by immunohistochemistry. In addition, most of them can also be detected by ELISA and flow cytometry.