When clinical studies find that there are potentially dangerous proteins on cells, researchers hope to shrink themselves into mini-surgeons, specifically eliminate problematic protein molecules, and keep healthy cells intact. In a recent new study, researchers from Stanford University in the United States discovered tools that can excise individual proteins from the surface of cells. The relevant research results were published online in the journal Nature on July 29, 2020.
Most existing treatments for individual proteins rely on interactions with specific activity regulation of the target protein, such as enzyme inhibition or ligand blockade. However, there are some treatment-related proteins whose activities are unknown or unavailable, so the strategies above cannot be used to treat some proteins. In addition, strategies involving protein degradation platforms such as proteolytic targeting chimeras and other platforms (such as dTAGs, Trim-Away, chaperone-mediated autophagy targeting, and SNIPERs) have been developed for proteins that are generally difficult to target. However, these methods require the use of intracellular protein degradation mechanisms, so they are limited to proteins containing intracellular domains, and ligands can bind to these domains and recruit necessary cellular components.
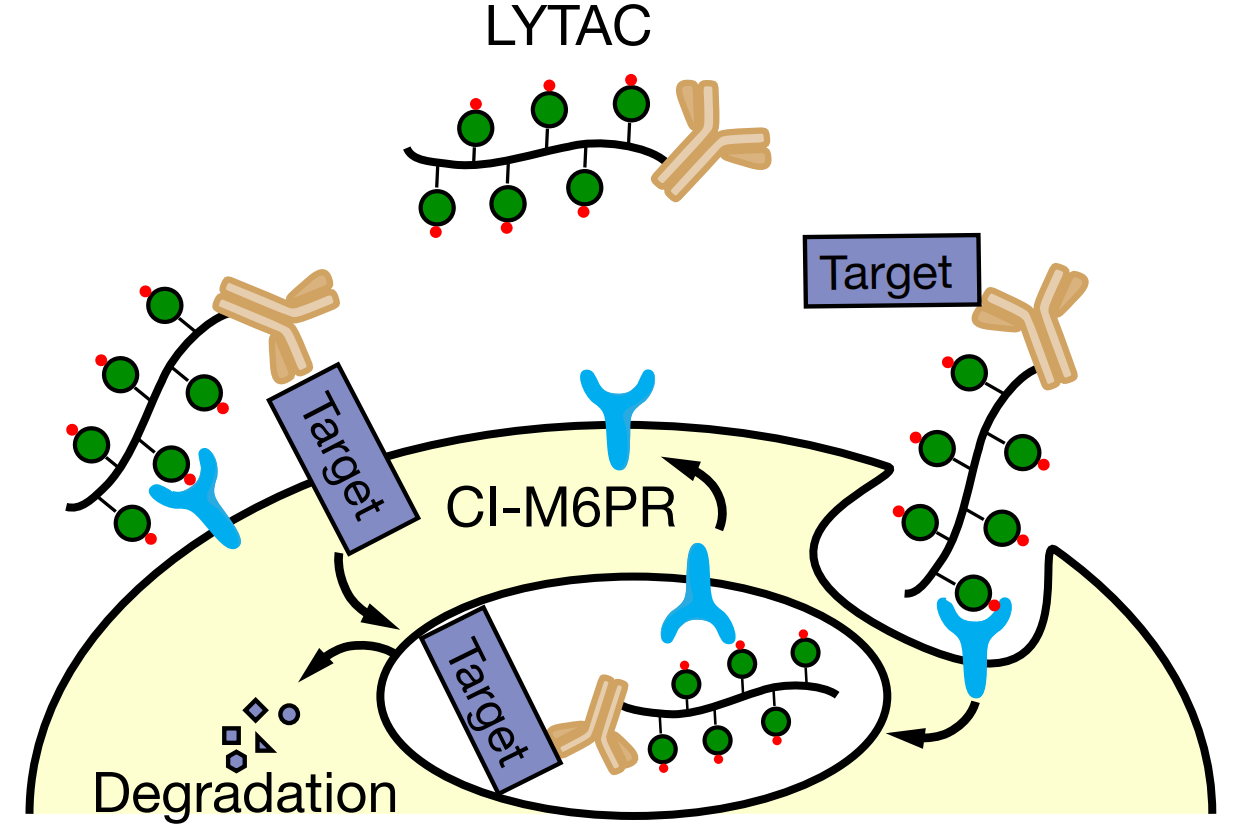
Studies have found that extracellular proteins and membrane-associated proteins account for 40% of all protein-coding genes and are key factors in cancer, aging-related diseases and autoimmune diseases. Therefore, the general strategy of selective degradation of these proteins may improve human health . In this study, the researchers used a conjugate that binds to the lysosomal shuttle receptor on the cell surface and the extracellular domain of the target protein to determine a targeted degradation strategy for extracellular and membrane-associated proteins. These researchers have developed a new class of molecules that can shuttle unwanted proteins from the cell surface or the surrounding environment to the lysosome, which is the cell compartment dedicated to protein degradation. These molecules are called lysosomal targeted chimeras (LYTACs).
Mechanism of action of lysosome targeted chimera (LYTAC)
Existing studies have shown that cation-independent mannose-6-phosphate receptor (CI-M6PR) is involved in the biochemical pathway that mediates the internalization of cell line cargo. LYTAC uses this mechanism to transport labeled targeting molecules to the lysosome for degradation. The LYTAC molecule consists of an oligosaccharide peptide group (which binds to the cell surface receptor CI-M6PR) and an antibody that binds to a specific transmembrane protein or extracellular protein. The antibody can also be replaced by a small protein binding molecule. When combined with CI-M6PR and target protein at the same time, the resulting complex is swallowed by the cell membrane to form a transport vesicle. This brings this complex to the lysosome, after which the target protein is degraded and the receptor CI-M6PR is recycled. By degrading treatment-related proteins, including apolipoprotein E4, epidermal growth factor receptor, CD71, and programmed death ligand, it proves that the platform has a wide range of effects.
The key to this tool’s function lies in its dual-function design. One side of the LYTAC molecule can be customized to bind to any protein of interest. On the other side of it is a short amino acid sequence, or peptide, inlaid with a sugar called mannose-6-phosphate. This sugar acts as a label for the cell. When a cell contains proteins that are sent to the lysosome for degradation, it adds this sugar to ensure they reach their destination. There are receptors on the cell surface that interact with this sugar. When they grab the LYTAC molecule and pull it into the cell, the labeled protein will also be dragged in with it. In the process of attaching this tag to the protein, LYTAC hijacks a natural cell shuttle mechanism designed to escort newly synthesized lysosomal proteins to their new home. However, lysosomal proteins are tough enough to survive in the presence of degrading enzymes encountered in lysosomes, and most proteins cannot do this, so those that are labeled proteins by the LYTAC method are usually destroyed. This selective degradation can help scientists study and treat diseases such as cancer and Alzheimer’s disease related to surface proteins by targeting and degrading proteins that play an important role. More interestingly, the tethered end of LYTAC can be anything that binds to proteins, such as antibodies or existing drugs, so in the future, many other proteins and diseases can be target attacked.
