There are two kinds of fat in the body, brown and white. White fat accumulates under the skin and is responsible for storing excess calories. Brown fat itself does not store calories and is mainly responsible for the decomposition of white fat that causes obesity, converting the latter into carbon dioxide, water and heat. The brown adipose tissue is brown, which is characterized by rich capillaries in the tissue. There are many small lipid droplets scattered in the fat cells. The mitochondria are large and rich, and the nucleus is round. This type of fat cell is called a multivesicular fat cell.
The Mystery of Thermogenesis of Brown Fat
Existing experimental results indicate that brown adipose tissue (BAT) plays a vital role in adaptive heat generation. Among them, adaptive heat generation is a physiological process in which energy is dissipated in response to changes in the environment such as cold and diet. In addition to the normal presence of BAT, in a cold environment, with β-adrenergic receptor agonists and other stimulation, brown-like multi-chamber beige / tan fat cells can be recruited in white adipose tissue (WAT) . The discovery of brown and beige fats that are metabolically active in adults makes this tissue the focus of human energy metabolism research. Because the study of the metabolic mechanism of brown fat can not only lead to the activation of enhanced glucose tolerance and insulin sensitivity in humans and mice through brown and beige / BRITE fat, but also potentially be used to combat obesity and its related metabolism disorder.
Previous studies have shown that the unique thermogenic ability of brown and beige / brite fats can be attributed to the expression of high-density mitochondria and uncoupling protein 1 (UCP1) in these thermogenic fat cells. Among them, UCP1 acts as a proton channel, located in the mitochondrial inner membrane, allowing protons in the mitochondrial membrane gap to re-enter the mitochondrial matrix without generating ATP. This process consumes energy and converts chemical energy into heat energy.
Traditional View of The Differentiation Of Brown Adipocytes
The traditional view of the differentiation of brown adipocytes and preadipocytes involves the activation of the adipogenesis / thermogenetic transcription cascade, including peroxisome proliferator-activated receptor gamma (Pparg), CCAAT / enhancer binding protein, PR domain containing 16 (Prdm16), peroxisome proliferation activation receptor γ, and co-activation factor 1α (PPARGC1A). This activation results in the expression of characteristic genes of fully differentiated brown fat cells, including fatty acid binding protein 4 (Fabp4), deiodinase 2 (Dio2), DNA cleavage factor A (Cidea) that induces cell death, and brown fat marker Ucp1 . Previous studies have found that UCP1 expression is restricted to the late stages of brown fat differentiation during routine in vitro differentiation. Therefore, in the process of brown fat differentiation, the regulation of UPC1 expression may be the key to induce differentiation.
Differentiation Mechanism of Brown Fat
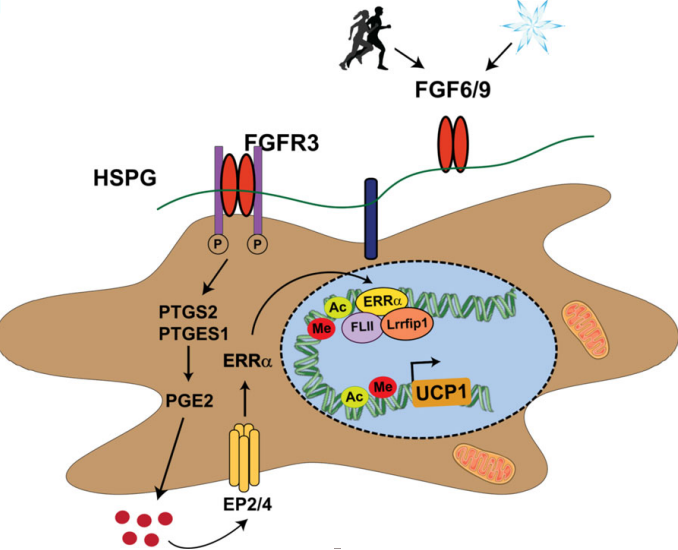
Based on this hypothesis, the researchers recently discovered factors that can induce Ucp1 expression in the mouse brown preadipocyte cell line through peptide library screening. The screening identified fibroblast growth factor 6 (FGF6) and FGF9 as effective inducers of Ucp1 expression. Contrary to the classical view of brown fat formation, researchers here found that FGF6 and FGF9 can induce high levels of Ucp1 expression in brown and white preadipocytes, regardless of fat formation. In contrast, UGF1 expression induced by FGF6- and FGF9 is mediated by stimulation of the biosynthesis of prostaglandin E2 (PGE2). Combining CRISPR-based chromatin immunoprecipitation (ChIP) and quantitative proteomics, reseacheres finally found a transcriptional regulatory complex that is consisted by the nuclear receptor estrogen-related receptor alpha (ESRRA or ERRA), transcription coactivator flightless I actin binding protein (FLII) and and leucine rich repeat interacting protein 1 (LRRFIP1). The complex can regulate the espression of Ucp1. Importantly, thermal stimuli can induce the expression of Fgf6 and Fgf9 in adipose tissue, such as cold exposure and exercise training. The absence of FGF9 in BAT impairs temperature regulation and reduces the heat production capacity of BAT. In contrast, overexpression of FGF9 in vivo can enhance the heat generation function of BAT. Therefore, FGF6 / 9 induces UCP1 expression, which in turn determines the thermogenic activity of brown adipocytes.
