The endoplasmic reticulum (ER) is a very important multi-functional organelle in eukaryotic cells. While completing the basic physiological functions, the endoplasmic reticulum becomes a pivotal platform for coordinated signal transduction with its large membrane structure. The endoplasmic reticulum is a site for lipid and sterol synthesis, maintenance of Ca2+ dynamic balance, protein synthesis and folding, and post-translational modification, as well as lipid and steroid metabolism. The endoplasmic reticulum cavity is a unique cell compartment capable of active Ca2+ transport, which is the compartment with the highest intracellular Ca2+ concentration. Under normal physiological conditions, Ca2+ is absorbed into the lumen of the endoplasmic reticulum by the cytoplasm through the calcium pump on the endoplasmic reticulum membrane, and is released into the cytosol by the IP3R and RyR channels, thereby maintaining the stability of free Ca2+ in the endoplasmic reticulum. Due to its protein folding and transport function, the endoplasmic reticulum has a large number of Ca2+-dependent molecular chaperones, such as calcium reticulin and glucose-regulated protein 78. The endoplasmic reticulum must strictly control Ca2 + levels to avoid Ca2+ imbalance leading to cell death. In addition, the endoplasmic reticulum plasma membrane (PM) acts as a barrier to free diffusion throughout the cell, connecting different organelles through a series of contact sites. A growing body of research indicates that ER-PM contact sites play an important role in maintaining Ca2+ homeostasis, signaling, and lipid regulation. Genetic or environmental damage can cause intracellular calcium homeostasis, oxidative stress, nutrient deficiencies, glycosylation inhibition, and protein misfolding, thereby disrupting endoplasmic reticulum function and inducing endoplasmic reticulum stress. Endoplasmic reticulum stress lead to the protein incorrectly folded and accumulated in the lumen of the endoplasmic reticulum. The cells pass through a series of signal network transduction pathways of PERK-eIF2α, IRE1–XBP1 and ATF6–CREBH to improve the correct folding ability of the protein, inhibit the production and accumulation of proteins, and accelerate the non-functionality and toxic protein degradation, triggering transcription of genes involved in endoplasmic reticulum stress and enhancing the self-repairing capacity of the endoplasmic reticulum. These reactions are collectively referred to as unfolded protein response (UPR). If the endoplasmic reticulum stress is too strong or persistent for too long, these reactions are not sufficient to restore endoplasmic reticulum homeostasis, which ultimately leads to apoptosis.
Endoplasmic Reticulum Stress and Disease
Endoplasmic Reticulum Stress and Osteoporosis
Osteoporosis (OP) is a common systemic metabolic disease in the elderly. It is characterized by bone pain, decreased bone strength, increased bone fragility, and degeneration of bone tissue. Osteoporosis is marked by low bone mineral density (BMD), which is associated with endoplasmic reticulum stress. The development of the pancreas and skeletal muscle system after birth is required to pass the PERK-eIf2α signaling pathway. PERK-eIF2α pathway can be activated by endoplasmic reticulum stress. ATF4 is a downstream target molecule of the PERK pathway, and ATF4 can stimulate cBFA1 to induce the expression of osteocalcin (OCN) in osteoblasts. These results indicating that endoplasmic reticulum stress is closely related to osteoporosis and bone development.
Endoplasmic Reticulum Stress and Cancer
Numerous studies have confirmed that endoplasmic reticulum stress is closely related to the occurrence and development of cancer. Many types of cancer cells rely on the high protein synthesis and folding ability of endoplasmic reticulum molecular chaperones to ensure the growth and spread of cancer cells. The microenvironment of hypoxia, redox imbalance, pH fluctuations and nutrient supply in tumor cell growth induces UPR. In addition, studies have found an increase in UPR signaling in tumor cells. Some key molecules in the endoplasmic reticulum stress response have been shown to be essential for tumorigenesis. For example, GRP78 /BiP protein is highly expressed in a variety of cancers, such as prostate cancer, breast cancer, lung cancer, melanoma, and colon cancer cells. GRP78 /BiP can strengthen tumors cell growth, proliferation and inhibition of tumor cell apoptosis by increasing the protein folding capacity of the endoplasmic reticulum.
Endoplasmic Reticulum Stress and Degenerative Neurological Diseases
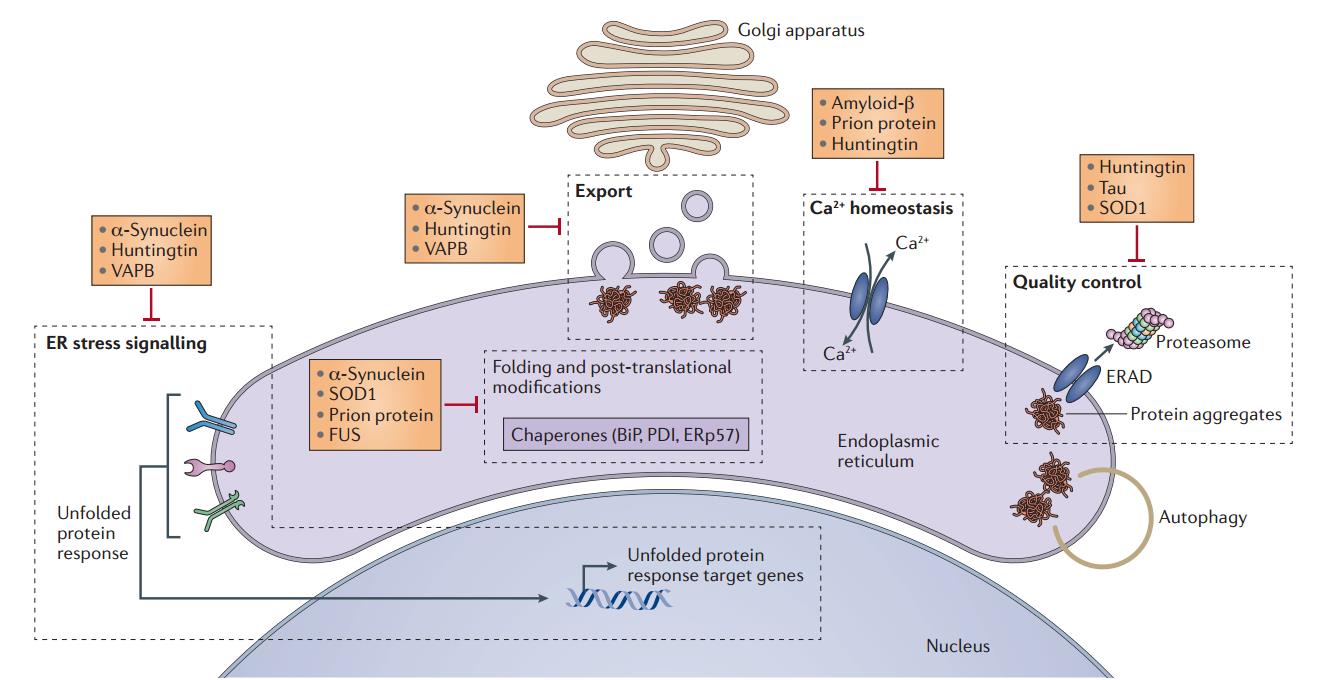
The pathogenesis of neurodegenerative diseases is complex and diverse. The study found that UPR is closely related to neurodegenerative diseases. Misfolded protein accumulation is a prominent feature of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. Accumulation of misfolded proteins causes endoplasmic reticulum stress and activates UPR. Long-term UPR can lead to cell death. This may be related to the pathogenesis of neurodegenerative diseases. For example, Alzheimer’s disease (AD) is the most common neurodegenerative disease, and its major pathological features are beta amyloid deposition to form neuroinflammatory plaques and tau hyperphosphorylation to form neurofibrillary tangles. Among them, the production of Aβ is a characteristic marker of AD. Aβ aggregation leads to senile plaque formation, synaptic loss, neurological dysfunction and neuronal death. The increasing and accumulation of Aβ is related to endoplasmic reticulum stress. Aβ can induce the release of Ca2+ from the endoplasmic reticulum into the cytosol while a large amount of Ca2+ in the plasma membrane and endoplasmic reticulum also promotes the increase in Aβ production by altering the metabolism of Aβ. In addition, many endoplasmic reticulum-related molecular chaperones such as GPR78 are Ca2+ binding proteins, and Ca2+ depletion in the endoplasmic reticulum can affect the function of these proteins and affect the synthesis and folding process of proteins, aggravating endoplasmic reticulum stress and causing cell damage.
