Mesothelin is a glycosylphosphatidylinositol (GPI)-anchored cell surface glycoprotein. Its biological function has not yet been clarified, but due to its limited distribution in normal tissues and high expression in some tumor tissues, it is expected to be used in tumor-specific treatment.
In existing studies of human epidermoid carcinoma cell lines expressing mesothelin, biodistribution analysis of mesothelin using radiolabeled monoclonal antibodies showed good localization and specificity for relevant tumors. So far, anti-mesothelin antibody-drug conjugates have been successfully used to treat mesothelin-expressing solid tumors, including ovarian cancer, pancreatic cancer, and malignant mesothelioma; and mesothelin targets chimeric antigen receptor (CAR) T cell therapy also showed significant antitumor effects. Therefore, targeted therapy and immunotherapy against mesothelin show promising application prospects.
Structure and Function of Mesothelin
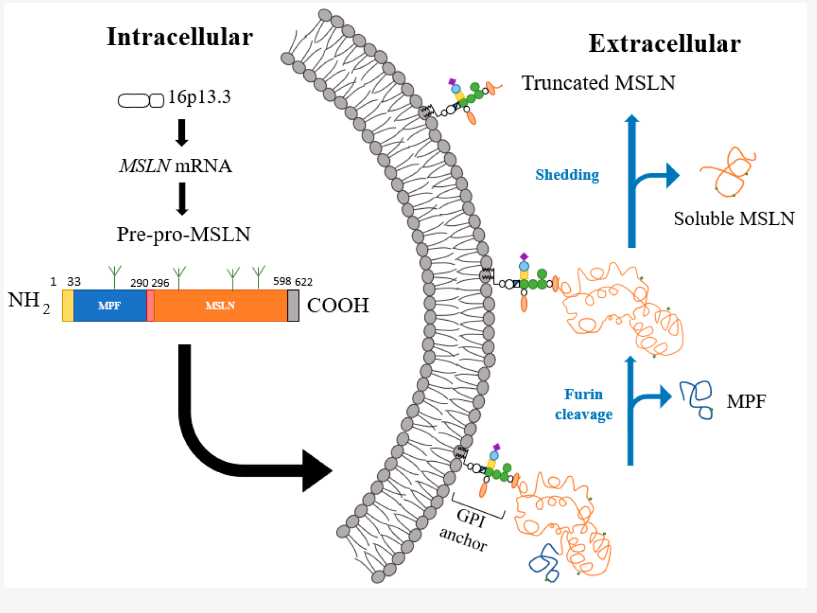
The mesothelin gene is located on chromosome 16p13. The mesothelin gene MSLN contains 17 exons. The cDNA is about 2138bp long, contains an open reading frame of 1884bp, and encodes a precursor protein of 628 amino acids (69 kDa). The precursor protein can be hydrolyzed by furin protease into two parts: mesothelin with a size of 40 kDa and megakaryocyte colony-stimulating factor (MPF) with a size of 31 kDa.
Like many GPI-anchored proteins, mesothelin is shed to generate soluble mesothelin-related peptides (SMRPs), which are commonly used as markers for the detection of malignant mesothelioma. Due to the presence of multiple proteases in the extracellular environment, mesothelin can be cleaved at different extracellular sites, and on cells expressing mesothelin, there are seven major cleavage sites near the membrane end, resulting in membrane-bound truncated mesothelin.
GPI-linked proteins are often involved in cell signaling and adhesion, so mesothelin may play a role in these biological processes. The exact role of mesothelin under normal physiological conditions is unknown. MSLN knockout mice have normal development and reproductive capacity, suggesting that mesothelin function is not essential for life. However, in MSLN knockout mice, the ultrastructure of the mesothelial membrane was affected, suggesting that mesothelin may play a role in shaping the tumor microenvironment. Compared with wild-type mice, the growth of intraperitoneal cancer cells in MSLN knockout mice was significantly reduced. Supplementation of mesothelin or MPF to stimulate the growth of MSLN knockout mice promotes lung cancer growth, which supports the role of mesothelin in tumor cell adhesion, migration and metastasis.
The Role of Mesothelin in Cancer
Mesothelin is expressed in many solid tumors, most commonly in mesothelioma, epithelial ovarian and pancreatic cancers, but also in lung and uterine malignancies and in cholangiocarcinomas. Mesothelin is highest expressed in ovarian cancer and mesothelioma, and higher expression correlates with advanced tumor stage and higher metastatic rates. In stage IV colorectal cancer, high mesothelin expression is directly associated with chemotherapy resistance, aggressiveness, and poor prognosis. In contrast, data from pancreatic cancer seem to suggest that, despite its expression, mesothelin is not associated with cancer aggressiveness. This suggests that mesothelin expression and its relationship with prognosis and tumor aggressiveness are tumor specific.
Studies have found that the interacting protein of mesothelin is CA125/MUC16, which is a member of the mucin family and is expressed in ovarian cancer and malignant mesothelioma. The interaction between CA125/MUC16 and mesothelin mediates heterotypic cell adhesion in vitro and is thus considered as a potential mechanism for peritoneal metastasis of ovarian tumors. The combination of CA125/MUC16 and mesothelin down-regulates DKK1 (Dickkopf-1, WNT signaling pathway inhibitor) through the SGK3/FOXO3 signaling pathway, thereby promoting migration. Blocking CA125/MUC16 and mesothelin binding restores DKK1 levels and prevents ovarian cancer metastasis.
Binding of soluble mesothelin to surface-anchored mesothelin triggers matrix metalloproteinase-7 (MMP-7) via the ERK1/2 (extracellular signal-regulated kinase), Akt, and JNK (c-Jun N-terminal kinase) signaling pathways expression leading to enhanced migration and invasion. Mesothelin overexpression in ovarian cancer cells had similar effects and was significantly associated with MMP-7 expression. Furthermore, mesothelin binding to CA125/MUC16 also triggers MMP-7 expression through the p38 MAPK pathway in pancreatic ductal adenocarcinoma.
In addition, pancreatic cancer cell lines overexpressing mesothelin significantly increased proliferation and accelerated cell cycle progression compared with mesothelin-silenced cell lines. Overexpression of mesothelin leads to constitutive activation of STAT3, leading to increased expression of cyclin E, cyclin E/cyclin-dependent kinase 2 (CDK2) complex, and faster G1-S transition.
