In recent years, monoclonal antibody therapy against immunological checkpoints has made breakthroughs in cancer treatment, especially in the treatment of various tumors such as melanoma, lung cancer, kidney cancer and bladder cancer. The mechanism of action is briefly described below. In cell immune, the immune response of T cells is regulated by complex inhibitory signals (also known as “immunization checkpoints”) to prevent uncontrolled immune responses or even autoimmune diseases. Among them, programmed death molecule 1 (PD-1) plays an important role in immunization checkpoints. PD-1 is expressed on the surface of T cells and belongs to the co-inhibitory molecule, playing a similar role as a “brake” in the immune system. The ligand for PD-1 includes PD-1 ligand (PD-L1) and PD-L2. PD-L1 is mainly induced on immune cells (such as tumor infiltrating lymphocytes) and epithelial cells (such as tumor cells), while PD-L2 is expressed only on APC cells. This means that PD-1 ligand PD-L1 is expressed on both tumor cells and tumor infiltrating lymphocytes, but not on antigen-presenting cells. Therefore, PD-1/PD-L1 inhibits T cell activation mainly in the tumors micro environment.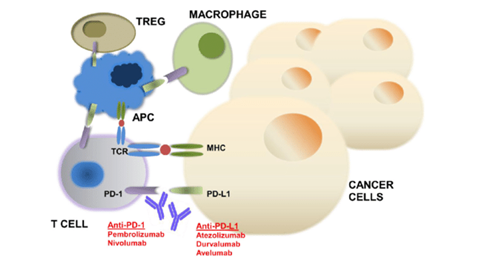 Figure 1. The function of PD-1/PD-L1 inhibitor
Figure 1. The function of PD-1/PD-L1 inhibitor
PD-1/PD-L1 inhibitor
Anti-PD-1 mAb mainly blocks the combination of PD-1 receptor and PD-L1 (B7-H1) or PD-L2 (B7-DC) to release T cell activity. The inhibition will promote the attack of activated T cells on tumor cells. Programmed death-1 (PD-1) and its ligand (PD-L1) inhibitors are immune checkpoint drugs, and the breadth, depth, and persistence of their responses show great potential in clinical. It is a hot spot in the research of tumor immunotherapy in recent years. The marketed nivolumab and pembrolizumab are PD-1 inhibitors. Nivolumab is a monoclonal antibody that binds to programmed death receptor-1 (PD-1) and blocks PD-1 by blocking the interaction between PD-1 and its ligands. Pathway-mediated immunosuppressive responses include anti-tumor immune responses. Therefore, the anti-tumor of cell immune is improved. In 2014, Nivolumab was approved by the US FDA for melanoma patients. In 2015, it was approved by the US FDA for the treatment of non-small cell lung cancer (NSCLC). It is the first lung cancer immunotherapy drug. Pembrolizumab is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligand and is an IgG4 kappa immunoglobulin. The drug is suitable for the treatment of unresectable or metastatic melanoma. In addition, PD-L1 is also a target of blocking the immune inhibitory. And its inhibitors atetolizumab, durvalumab and avelumab have been approved for the treatment of urothelial carcinoma.
Side effects of PD1/PDL1 immunosuppressive agents
Although the treatment of immunosuppressive agents has brought new hopes for the treatment of cancer, the toxicity of these drugs has attracted people’s attention. Immunosuppressive therapy can kill the tumor cell by removing inhibition of body’s own immune system. However, the enhanced immune system will induce excessive immune in body, so the adverse events associated with the immunological checkpoint inhibitor include almost all organs. During inflammatory processes, PDL1 is critical for maintaining an immunosuppressive environment, and any tissue stimulated by interferon expresses PDL1 to alleviate the damage caused by T cells during inflammation. If a PDL1 inhibitor is administered to a patient, the system’s immunosuppression will be ineffective, which increases the likelihood of inflammatory damage surrounding the tumor. Since PDL1 expression is in a wider scope, treatment with a PDL1 inhibitor will result in a higher incidence of side effects and autoimmune events, while PD1 inhibitors primarily affect T cells and therefore do not cause unwanted autoimmune events in the body.
Creative Diagnostics offers PD1 / PDL1 / PDL2 related antibodies and conjugates validated for use in a variety of common applications including WB, FC, IHC, ICC, IF, IP, and more.
