During 2001 scientists extracted human metapneumovirus (hMPV) from the nasopharyngeal secretions of hospitalized children suffering from respiratory infections. Human metapneumovirus (hMPV) has spread worldwide since 1950 and remains a persistent respiratory pathogen. Human metapneumovirus targets children and elderly populations but adults and immunocompromised individuals can also contract the virus. The clinical presentation of patients ranges from mild respiratory infections to severe bronchiolitis and pneumonia but researchers have not yet fully determined the complete pathogenesis. Currently the market lacks both vaccines and specific drugs for hMPV and treatments focus predominantly on alleviating symptoms.
What is hMPV?
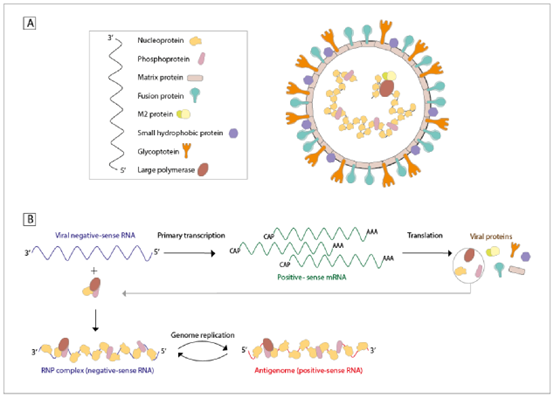
Human metapneumovirus occupies the genus Metapneumovirus within Pneumovirinae subfamily of Paramyxoviridae family while human respiratory syncytial virus shares this subfamily but belongs to a different genus. The virus hMPV consists of single-stranded negative-sense RNA and measures approximately 200nm in diameter. The viral RNA binds tightly to nucleoprotein and surface glycoproteins F, G, and SH create spikes measuring 13-17nm. According to the sequence of G or F, hMPV can be divided into type A and type B, and can be further divided into 4 subtypes: A1, A2, B1, and B2. Research indicates that multiple hMPV types are capable of spreading at the same time in particular locations while the dominant strain varies every 1 to 3 years. Cross-neutralizing antibody tests and hamster and non-human primate comparisons revealed strong antigenicity correlation between hMPA type and type B. HMPV infection manifests primarily through upper and lower respiratory tract infections while lower respiratory tract infections prevail as the most frequent presentation which may worsen pneumonia, bronchiolitis and acute asthma.
Figure 1. Structure, genetic material, and replication cycle of human metapneumovirus (hMPV). (Gálvez NMS, et al.; 2021.)
Mechanism of hMPV Infection of Host Cells
The hMPV genomic RNA is about 13kb long. Except for the gene order and the deletion of some non-structural genes, the hMPV and RSV genomes are very similar. The hMPV genome encodes 9 proteins, N is a nucleoprotein, P is a nuclear envelope phosphorylated protein, M is a non-glycosylated matrix protein, F is a fusion protein, M2-1 is a transcription elongation factor, M2-2 is an RNA synthesis regulator, SH is a small hydrophobic surface protein, G is the main adhesion protein, and L is the main polymerase subunit. F, G and SH are viral surface glycoproteins. Before hMPV fuses with the host cell membrane, the viral surface glycoproteins bind to the host cell membrane surface receptors, but not all surface glycoproteins are necessary for entry into the cell. Mutants with G or SH deletions can replicate and proliferate efficiently in hamster and non-human primate models. F can independently induce fusion, but G and SH cannot, indicating that F plays a key role in viral infection. In addition, both F and G of RSV can induce neutralizing antibodies, while only F of hMPV can induce neutralizing antibodies, indicating that hMPVF is an important target for vaccine and drug development.
During the intracellular process, F is initially translated into an inactive precursor F0, which acquires fusion ability through proteolysis. The RSVF0 precursor is recognized and cleaved twice by proteases, but the hMPVF0 precursor is only cleaved once. The F1 and F2 subunits produced by hMPVF cleavage remain covalently linked through disulfide bonds. Mature hMPVF is a F1+F2 homotrimer in a metastable prefusion conformation (prefusion, Pre). After receiving the activation signal, the high-energy state of PreF undergoes a tertiary structural rearrangement, extending the hydrophobic fusion peptide at the N-terminus of the F1 subunit and inserting it into the host cell membrane. Then, this intermediate shrinks itself, pulling the host cell and virus membranes closer, and membrane fusion occurs, at which time F is in a highly stable postfusion conformation (postfusion, Post). The F of RSV and hMPV have approximately 30% sequence homology, and at least 2 RSVF antigenic sites are the same as hMPVF (SiteⅢ and SiteⅣ), but the induced antibody responses are different. The neutralizing activity of RSV in human serum is mainly mediated by PreF-specific antibodies, while the neutralizing activity of most hMPV is mediated by both PreF and PostF antibodies. The immune effect of RSVPreF is better than PostF, but there is no conclusion whether hMPVPreF is better than PostF. Studies have shown that RSVPreF-specific antibodies are more common in infected or vaccinated humans and mice, while the level of hMPVPreF-specific antibodies is lower than that of PostF.
Research Progress of hMPV Vaccines in the Clinical Stage
Currently, no hMPV vaccine has been marketed. There are currently 6 vaccines in the clinical stage, namely: RSV and hMPV combined vaccine IVX⁃A12, hMPV and PIV3 combined vaccine mRNA⁃1653, RSV and hMPV combined vaccine mRNA⁃1365, RSV, hMPV and PIV combined vaccine SP0256, RSV and hMPV combined vaccine VXB⁃241, and live attenuated vaccine rHMPV⁃Pa.
IVX⁃A12
IVX⁃A12 is designed based on protein two-component virus-like particles (VLPs) I53⁃50, and the two VLPs display RSVPreF antigens (DS⁃Cav1) and hMPVPreF antigens, respectively. The main purpose of the IVX⁃A12 Phase I clinical trial (NCT05664334) is to evaluate the safety and immunogenicity of a single vaccination of two VLPs at different doses and ratios, with or without MF59®, in healthy elderly subjects. Interim data showed that all dose groups were well tolerated and induced immune responses against RSV and hMPV with or without adjuvants, with no immune interference between antigens. The highest geometric mean titers of neutralizing antibodies induced by IVX⁃A12 for hMPV⁃A and hMPV⁃B were approximately 3300 assay units/mL and 23900 assay units/mL, respectively, while those in the placebo group were approximately 900 assay units/mL and 11500 assay units/mL, respectively. Subjects will continue to be followed up to obtain immunogenicity data for 6 months. The purpose of the Phase II clinical trial (NCT05903183) is to evaluate the safety and immunogenicity of a single dose of MF59®IVX-A12 in healthy elderly people, and to investigate long-term safety and immune persistence. Currently, the results of the trial have not been announced.
mRNA⁃1365
mRNA⁃1365 adopts the mRNA technology route and is in the recruitment stage of Phase I clinical trial (NCT05743881). It mainly examines safety and immunogenicity. The subjects are infants aged 5 to 24 months. The test results have not yet been announced.
SP0256
SP0256 is an mRNA respiratory virus combined vaccine designed for the elderly. The antigens mainly include RSV, hMPV and PIV. At present, the clinical trials of RSV and hMPV combined vaccines conducted by this vaccine (NCT06134648, NCT06237296 and NCT06583031) are still in progress, studying the effects of different antigen doses, lipid nanoparticle components and vaccination doses on safety and efficacy.
VXB⁃241
In antigen design, VXB⁃241 uses regions 1 and 2 of the heptapeptide repeat sequence of human immunodeficiency virus type Ⅰ gp41 protein to form a molecular clamp, which binds to the recombinant viral glycoprotein to replace the C-terminal transmembrane/cytoplasmic region to form a six-helix bundle including the viral fusion protein, thereby restricting the viral glycoprotein trimer to Pre. Currently, the vaccine has been launched in Phase I clinical trials (NCT06556147) to study the safety and immunogenicity of different doses of antigens, and the results have not yet been announced.
Others
The antigen of mRNA⁃1653 is wild-type hMPV mRNA. Phase I clinical trials (NCT04144348 and NCT03392389) showed that the vaccine was well tolerated in adults and infants, and the level of neutralizing antibodies was increased. A research group is committed to developing a live attenuated hMPV vaccine. They first constructed a genetically stable live attenuated vaccine backbone rHMPV-SHs, and then replaced the P in it with the corresponding gene of avian metapneumovirus (AMPV) to obtain the live attenuated vaccine rhMPV-Pa. However, the study was terminated because the vaccine was over-attenuated in hMPV seronegative children.
| Cat.No | Product Name | Source/Host | Application | |
| DMAB-CLS25015 | Magic™ Anti-hMPV M Protein Mab, clone 2242 | Mouse | LFIA(Cap) | Inquiry |
| DMAB-CLS25016 | Magic™ Anti-hMPV M Protein Mab, clone 2243 | Mouse | LFIA(Det) | Inquiry |
| DMAB-CLS25017 | Magic™ Anti-hMPV NP Mab, clone 4E3C | Mouse | LFIA(Cap) | Inquiry |
| DMAB-CLS25018 | Magic™ Anti-hMPV NP Mab, clone 4E3MB | Mouse | LFIA(Det) | Inquiry |
| DMAB-CLS25019 | Magic™ Anti-hMPV NP Mab, clone 5C9H | Mouse | LFIA(Cap) | Inquiry |
| DMAB-CLS25020 | Magic™ Anti-hMPV NP Mab, clone 5C9MB | Mouse | LFIA(Det) | Inquiry |
| DMAB-CS23171 | Magic™ Anti-HMPV Mab, clone B6203 | Mouse | LFIA (Cap) | Inquiry |
| DMAB-CS23172 | Magic™ Anti-HMPV Mab, clone B6202 | Mouse | LFIA (Det) | Inquiry |
| DAG-WT646 | Recombinant hMPV M Protein | E. coli | LFIA, CLIA | Inquiry |
| DAG-WT617 | Recombinant hMPV NP | E. coli | LFIA, CLIA | Inquiry |
| DAGC321L | hMPV 9 Type A1 Lysate | LLC-mk2 cells | WB, DB | Inquiry |