The tumor microenvironment is composed of tumor cells and non-tumor cells (including fibroblasts, vascular and lymphatic endothelial cells, various immune cells) and extracellular components (such as extracellular matrix, cytokines, inflammatory factors, chemokines, etc.) in tumor tissues. Factors, etc.) interact to form a dynamic and complex network structure. Among them, chemokine receptors can participate in a variety of physiological and pathological processes after binding to their ligands, such as cell growth, development, differentiation, apoptosis, tissue damage and repair, etc. Chemokine receptors and their ligands can not only play an anti-tumor role in tumors by chemotaxis, activating immune cells or inhibiting vascular proliferation, but can also stimulate tumor growth, chemoattract tumor cells, and promote tumor blood vessel growth and promote the degradation of extracellular matrix to achieve the effect of promoting tumor growth, invasion and metastasis.
Figure 1. CXCL13/CXCR5-associated immune activities across tissues.
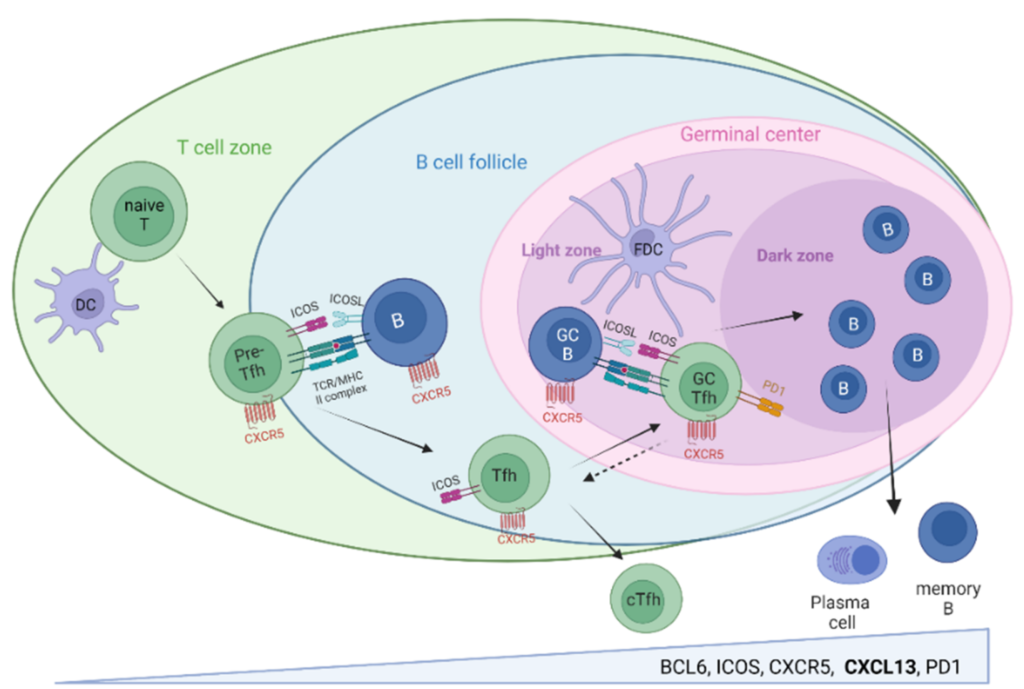
Biological Characteristics of CXCR5/CXCL13
C-X-C motif chemokine ligand 13 (CXCL13) is an important member of CXC-like chemokines, mainly expression and secretion of T lymphoid follicular helper cells, dendritic cells and part of the mesenchyme cells in secondary lymphoid tissue. CXCL13 is the only ligand of the G-protein coupled receptor family CXCR5. The CX-CR5/CXCL13 axis plays a pivotal role in the progression of many cancers. CXCL13 can promote tumor growth and invasion through the PI3K/AKT signaling pathway, or enhance anti-tumor immune responses by increasing tumor immune localization. Common CXCR5/CXCL13 axis-related immune cells include B cells, macrophage M1, NK cells, CD8+T cells, follicular helper T cells (THF), γδT cells, and those expressing CX-CR5 CD8+T cell subset, called follicles Cytotoxic T cells (Tfc).
The Role of CXCR5/CXCL13 in Different Tumors
The role of CXCR5/CXCL13 axis in solid tumors
Increased expression of CX-CR5/CXCL13 is associated with tumor occurrence and development in breast cancer, lung cancer, gastric cancer, clear cell renal cell carcinoma, and osteosarcoma. Studies have shown that in HER2+ breast cancer patients, increased CXCL13 expression has a better survival rate. Studies on female mice suggest that CX-CL13 has an inhibitory effect on tumor growth, which may be related to the CXCR5/ERK signaling pathway. This has important implications for immunotherapy in triple-negative breast cancer patients. CXCR5 and CXCL13 may be involved in the occurrence, metastasis and recurrence of advanced colon cancer through the PI3K/AKT signaling pathway. In mouse gastric cancer, CD40 can upregulate the chemokine receptor CXCR5, thereby inhibiting MDSC recruitment and T cell proliferation and promoting tumor immune escape. Bioinformatics analysis showed that low expression of CXCL13 indicates a good prognosis in ovarian cancer. DNA methylation-dependent down-regulation of CXCL13 may promote the occurrence and development of cervical cancer. In addition, CXCL13 promotes the invasion and metastasis of pancreatic ductal carcinoma by activating the ERK1/2 pathway and increasing the expression of ETV4.
The role of CXCR5/CXCL13 axis in hematological malignancies
Chemokine receptors affect the migration and homing of malignant lymphocytes in the blood tumor microenvironment, and can promote the growth and drug resistance of tumor cells. Among hematological malignancies, especially chronic lymphocytic leukemia (B-CLL) and acute lymphoblastic leukemia (B-ALL), there is important evidence that the expression of CXCR5/CXCL13 in PCNSL tumor tissue is 100%. In addition, elevated serum CXCL13 levels are associated with an increased risk of B-cell non-Hodgkin lymphoma (NHL) in HIV-infected patients. The p53 deletion may participate in the organ invasion and chemotherapy resistance of multiple myeloma through the miR-19a/CXCR5 pathway. Bone marrow mesenchymal stem cells express high levels of the chemokine CXCL13 in the bone marrow microenvironment, which affects the invasion, drug resistance and proliferation of bone marrow mesenchymal stem cell lines U266 and LP-1 through the CXCL13-mediated signaling pathway. In addition, the CXCL13 pathway also up-regulated the mRNA and protein expression levels of BTK, NF-κB, BCL-2 and MDR-1 in U266 and LP-1 cells, suggesting CXCR The 5/CXCL13 axis may provide new insights into the immunotherapy of multiple myeloma.
Applications Related to CXCR5/CXCL13 Axis and Tumor Immunotherapy
CXCR5 /CXCL13-B Cells and Tumor Immune
B cells through the use of B cell receptors (BCR) to identify the function of APC, which is very important for the stable state of exogenous antigen and its own antigen to maintain the body’s immune system. Intratumoral CXCR5+ tumor-infiltrating lymphocytes (TILs) were found in chronic inflammatory breast cancer, mainly B cells, and their abundance was significantly correlated with CXCL13 gene expression, suggesting that CXCL13 may promote and guide the infiltration and migration of B-TILs in the tumor. CXCR5/CXCL13 not only participates in the occurrence and development of tumors, but also forms a network structure with various cells in the tumor microenvironment to exert anti-tumor immune effects. B cells play an important role in humoral immunity. For example, in patients with colorectal cancer, increased intratumoral CXCL13 is associated with abundant T cell and B cell tumor infiltration and prolonged patient survival. Injecting CXCL13 into the colon submucosa of mice containing colorectal cancer can effectively prevent tumor growth. TABs (TABs) recruited by Th-CXCL13 cells in patients with nasopharyngeal carcinoma induce plasma cell differentiation and immunoglobulin production through the interaction of IL-21 and CD84, which may predict better survival rate. In addition, CD4+T cells can affect the infiltration of B lymphocytes in HPV+ head and neck squamous cell carcinoma through the CXCL13/CXCR5 axis.
CXCR5/CXCL13-T cells and Tumor Immunity
CD8+T cells are considered to be the main cytotoxic lymphocytes with anti-tumor effects. The occurrence of tumors is related to the functional exhaustion of CD8+T cells. In chronic viral infection and follicular lymphoma, CXCR5+CD8+T cells have stronger pro-inflammatory functions than CXCR5-CD8+T cells. Studies have found that CXCR5+CD8+T cells are an important CD8+T cell subset in colorectal tumors and have the potential to promote anti-tumor immunity. The recruitment of anti-tumor immune cells depends largely on the expression of chemokine receptors. In colorectal cancer, the frequency of CCR2-expressing and CXCR5-expressing cells was significantly down-regulated in CD4+ and CD8+ T lymphocyte populations, respectively, suggesting T cells anti-tumor function is reduced. Studies have shown that CXCR5+CD8+T cells are abundantly infiltrated in gastric cancer, muscle-invasive bladder cancer, serous ovarian cancer, head and neck squamous cell carcinoma, and pancreatic cancer, suggesting a good prognosis.