TB has continued to be a major human health threat throughout different centuries. The bacterium Mycobacterium tuberculosis (Mtb) causes tuberculosis which remains a persistent threat to human health by infecting millions of people annually. The world continues to fight TB because this infectious disease kills more people than any other infectious disease despite having vaccines and antibiotics and worldwide knowledge about it. Why? Because Mtb is not just another pathogen—it’s a master of immune evasion.
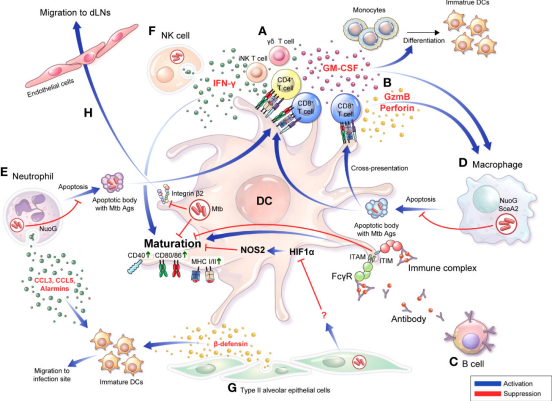
Figure 1.Bidirectional interactions between DCs and diverse cells are involved in the TB pathogenesis and protective response.(Sources:Kim H, et al.; 2022)
Recent research has shed light on a fascinating battleground between Mtb and one of our most powerful immune cells—the dendritic cell (DC). These cells are the immune system’s intelligence officers, detecting invaders, processing their molecular “fingerprints,” and presenting them to T cells to trigger a precise immune response. But Mtb has evolved a sophisticated arsenal to sabotage these defenders from within.
Let’s step into this microscopic battlefield to see how Mtb manipulates dendritic cells and what scientists are doing to fight back.
The Commanders of Immunity: Dendritic Cells
Dendritic cells sit at the frontline of the immune system. In their immature form, they patrol tissues, sampling their surroundings for pathogens. Once they detect danger, they mature and migrate to lymph nodes, where they “present” processed antigens via molecules known as MHC (major histocompatibility complex) to T cells. This antigen presentation sparks the adaptive immune response, enlisting an army of killer and helper T cells.
There are several subtypes of DCs—each with unique tasks.
- cDC1 specialize in “cross-presentation,” displaying viral or bacterial peptides to CD8⁺T cells.
- cDC2 focus on activating CD4⁺ helper T cells that coordinate immune responses.
- pDCs are the body’s antiviral sentinels, releasing large quantities of interferons to sound the alarm during infections.
When working properly, DCs are extraordinarily effective. But during TB infection, things don’t go according to plan.
Mtb’s First Encounter: Hijacking Recognition
As Mtb enters the lungs, it immediately encounters DCs and macrophages. These cells recognize the invader using pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and C-type lectin receptors. However, Mtb uses these very recognition systems against the host.
For example, Mtb lipoproteins such as Rv1016c bind to TLR2 receptors. Instead of triggering a strong immune response, this interaction dampens MHC-II molecule expression and interferes with antigen processing. The result? Weakened activation of CD4⁺T cells and prolonged bacterial survival.
Manipulating Maturation: Keeping Dendritic Cells in Check
Dendritic cell maturation is crucial for effective immune defense. Mature DCs express higher levels of costimulatory molecules and secrete cytokines that guide T-cell activation. Mtb cleverly tweaks this process.
Some Mtb proteins promote DC maturation—such as ESAT-6 and RpfE—which stimulate receptors like TLR2 and TLR4, encouraging Th1 and Th17 immune responses. However, many other bacterial components do the opposite.
For instance:
- Alpha-crystallin 1 (Acr1), a latency-associated protein, prevents DCs from maturing, keeping them in an “immature” state unable to activate T cells.
- ManLAM, a major cell wall component, binds to DC-SIGN receptors and triggers IL-10 overproduction—a cytokine that suppresses immune activation.
- Hip1, an Mtb hydrolase, cuts immune-stimulating proteins into less active forms, silencing DC responses.
- MPT64, another virulence protein, can even reprogram immature DCs into myeloid-derived suppressor cells that block T-cell proliferation.
It’s as if Mtb is cutting the power supply to the immune communication network—DCs see the intruder but can’t send the message.
Sabotaging the Cellular Machinery: Phagosomes and Autophagy
Once Mtb is engulfed by a dendritic cell, it should be trapped inside a deadly compartment called a phagolysosome—an acidic, enzyme-filled chamber designed to destroy bacteria. Yet Mtb has evolved to survive this fate.
It produces enzymes like PknG and PtpA that interfere with phagosome maturation and acidification, essentially disarming the cell’s digestive system. Another protein, EsxH, blocks an essential cellular sorting complex (ESCRT), crippling antigen processing and preventing T-cell activation.
Mtb disrupts autophagy which is the cellular recycling system that usually removes pathogens from cells. The bacterial proteins PE_PGRS47 and SapM block lysosome fusion with autophagosomes to establish a protective environment for Mtb replication. The bacterium survives inside host cells through its ability to block all cellular pathways that clean up pathogens.
Cutting the Communication Lines: Blocking Antigen Presentation
Mtb uses its glycoproteins Rv1860 and PPE18 to decrease MHC-II expression and CD40 and CD86 costimulatory molecule production in dendritic cells. The absence of these “badges” prevents dendritic cells from showing antigents to T cells which results in their inability to activate.
The bacterium releases its antigens from infected cells to prevent them from becoming MHC molecule display candidates. The bacterium employs a deceptive method to eliminate all evidence from cells before the immune system discovers its presence.
Turning the Tables: What Scientists Are Doing
Scientists have used their knowledge of molecular chess games to create new vaccine and therapeutic approaches which help restore or boost DC function.
Multiple vaccine candidates focus on attacking the pathways which cells use to present antigens:
- The recombinant BCG strain VPM1002 contains Listeria proteins which help cells break out of phagosomes while boosting their ability to show antigens.
- lMTBVAC, a live attenuated vaccine with specific gene deletions, enhances DC activation and boosts both Th1 and Th17 responses.
- lSubunit vaccines combining ESAT-6, Ag85B, and EsxH have been shown to induce durable T-cell immunity by improving antigen display efficiency.
By designing vaccines that “teach” DCs to resist Mtb’s manipulative tactics, researchers hope to outsmart the pathogen at its own game.
The Road Ahead: Decoding the Mtb–DC Dialogue
The world continues to report more than 10 million new TB cases annually despite achieving significant progress during the last few decades. Scientists need to keep studying the molecular communication between Mtb and dendritic cells to achieve the WHO’s 2035 target for TB elimination.
Future studies will likely focus on:
- Discovering new Mtb proteins that influence DC signaling.
- Developing small molecules or adjuvants that enhance DC maturation and antigen presentation.
- Exploring how microRNAs and metabolic pathways shape DC responses during infection.
The more we learn about how Mtb disarms our immune system, the better equipped we’ll be to design next-generation vaccines and therapies that can turn the tide against this ancient foe.
Conclusion
The history of tuberculosis involves the development of survival strategies through which the bacteria adapted to human environments. Mycobacterium tuberculosis has developed its immune cell manipulation skills during thousands of years of evolution by targeting dendritic cells which function as immune system connectors between innate and adaptive responses. Mtb maintains its human body residence through its ability to disable antigen presentation and stop autophagy and disrupt immune system communication.
Scientific research into Mtb’s immune evasion mechanisms leads to developing new treatment possibilities. Our understanding of Mtb’s dendritic cell evasion strategies enables us to develop countermeasures which turn defense weaknesses into powerful advantages. The battle against tuberculosis continues but we now possess the ability to read the enemy’s strategic plans.