Introduction of Golgi Bodies
Golgi is a highly polar organelle composed of many flat vesicles. It is often distributed between the endoplasmic reticulum and the cell membrane. It is slightly arcuate or hemispherical and has a certain polarity. The convex surface of the flat capsule is close to the nucleus or endoplasm net, called the generating surface or the immature surface, is opposite to the concave side facing the cell membrane side, called the secretory surface or the mature surface. Its main function is to process, classify and package the proteins synthesized by the endoplasmic reticulum, and then send them to specific parts of the cells or to the cells. Many studies have found that Golgi is involved not only in the secretory process of cells, but also in the post-translational modification and hydrolysis of proteins of protein glycosylation. Within neurons, the Golgi apparatus is involved in the cis, trans, and synaptic transport of many endogenous and exogenous proteins. Therefore, a certain or extensive function of the Golgi apparatus can cause abnormalities in protein and lipid transport, and even neuronal dysfunction, leading to disease.
Golgi apparatus is a highly dynamic organelle involved in the processing and classification of lipids and proteins. The maintenance of its morphology depends on the cellular transport process and the composition of many proteins. The morphology of the Golgi can change under different physiological conditions, such as cell mitosis, growth or metabolic requirements, and the Golgi changes in cells during these processes are reversible. When the drug-induced microtubule injury is caused by nocodazole or colchicine, the Golgi apparatus also reversibly disperses or fragments. In the case of pathological conditions such as impaired endoplasmic reticulum function, disruption of intracellular trafficking pathways, abnormal lipid metabolism, stress state, DNA damage, and activation of apoptotic pathways, the Golgi apparatus may also change. However, this change is generally irreversible and can even accelerate or cause cell death associated with Golgi and neurodegenerative diseases.
Golgi and neurodegenerative diseases
As aging progresses, the prevalence of neurodegenerative diseases is also rising. Golgi is increasingly being considered and studied as an important organelle. In neurons of neurodegenerative diseases, Golgi has undergone morphological changes such as cystic dilatation, rupture, reduced number, reduced volume, and reduced vesicles and adjacent vesicles associated with the rough endoplasmic reticulum, or aggregate at the periphery of the nucleus or cytoplasm. Among them, Golgi fragmentation is a typical feature of neurodegenerative diseases, and different mechanisms may be involved in different neurodegenerative diseases. Many studies have shown that the mutant copper-zinc superoxide dismutase mSOD1, β-amyloid (Aβ), alpha-synuclein, stathmin and Tau proteins are associated with Golgi fragmentation and neuronal degeneration.
ALS and mutation SOD1
SOD1 is an antioxidant enzyme that catalyzes the conversion of O2 to H2O2 and maintains the homeostasis of intracellular reactive oxygen species for detoxification purposes. Studies have found that the accumulation of insoluble proteins found in patients with SALS and FALS is immunoreactive SOD1, and its misfolding and protein aggregation are closely related to ALS. The SOD1 gene after mutation induces excessive free radicals. The toxic effect of free radicals, and mutational SOD1 aggregation, forms a high molecular weight insoluble complex, which ultimately leads to the death of motor neurons.
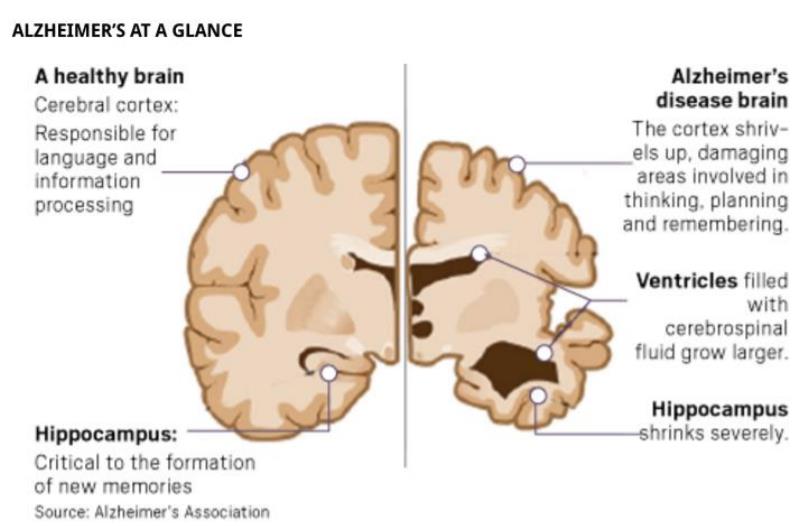
AD and Aβ, Tau
AD is a common neurodegenerative disease characterized by progressive dementia in the elderly. One of the typical pathological features of AD is the formation of a large number of senile plaques (SP), mainly caused by Aβ accumulation. Aβ in the brain is a polypeptide which contains 39-43 amino acid . It is produced by proteolysis of amyloid precursor protein (APP). As a transmembrane protein, APP is widely distributed in various tissues in the body, and has the highest expression in the brain. The normal operation of the Golgi apparatus is required for the transport, formation, classification and processing of APP and its secretase. The route of APP is from the endoplasmic reticulum to the Golgi body and then to the plasma membrane. For example, the formation of APP can produce Aβ, which may occur in the late Golgi and secretory pathways. If the Golgi transport function is impaired, it may affect the normal transport of APP and cause Aβ accumulation. At the molecular level, accumulation of Aβ causes Ca2+ influx, the process will activate calpain, and then increases the cleavage of P25-P35. Finally, P25 activates cdk5. Activated Cdk5 phosphorylates GRASP65, which ultimately leads to the fragmentation of the Golgi.
The typical pathological feature of AD is the formation of eurofibrillary tangles (NFTs), the main component of which is hyperphosphorylated tau protein. Tau protein is a microtubule-associated protein with the highest neuron content. Its main function is to promote microtubule synthesis and stabilize microtubules. It plays a role in maintaining the cytoskeleton and acts as a transprotant pathway for organelles, vesicles, proteins and signaling molecules, mostly localized in neuronal axons. Interesting, studies have found that the increase in Golgi fragmentation is age-related and is associated with hyperphosphorylation of Tau protein. Further studies find that Golgin-84 triggers hyperphosphorylation of the Tau protein by activating cdk5 and an extracellular signal-regulated kinase, which in turn induces Golgi fragmentation. Eventually triggers neuronal death and induces AD.
