The three common approaches to treating cancer are surgical removal, radiation therapy, and chemotherapy. Over the years, cancer immunotherapies such as immune checkpoint therapy have emerged and attracted much attention. This type of therapy treats cancer by activating the body’s immune system, with relatively few side effects and relatively better anti-cancer effects. In addition, for certain cancers, this type of therapy can significantly improve the long-term survival rate of patients, and may even achieve a complete cure, even in the advanced stages of cancer.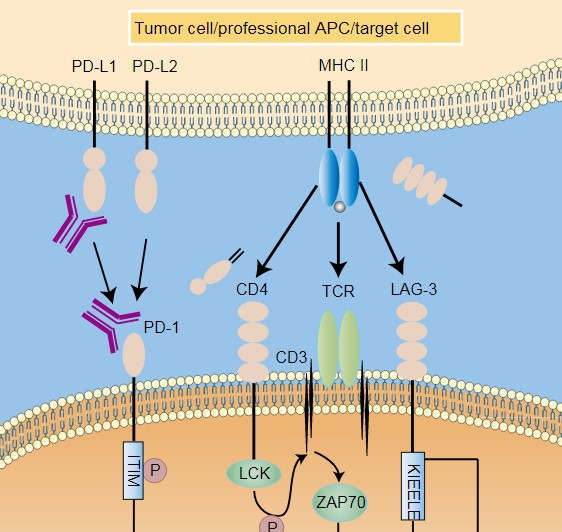
Immune checkpoints refer to some inhibitory signaling pathways that exist in the immune system, such as the PD-1/PD-L1 pathway and the CTLA-4 pathway. Under normal circumstances, in order to prevent activated T cells from destroying normal human cells, the immune system can control the activation of T cells by activating immune checkpoints such as PD-1 / PD-L1 to prevent T cells from erroneously attacking normal cells. However, cancer cells specifically recognize PD-1 on the surface of T cells by expressing the surface protein PD-L1, stealing this control mechanism, thereby activating immune checkpoints to suppress the immune activity of T cells, which will cause cancer cells to escape immune recognition and grow rapidly. This opens up a whole new line of immunotherapy ideas for cancer treatment-blocking immune checkpoints such as CTLA-4 or PD-1 through immune checkpoint inhibitors, preventing cancer cells from stealing this control mechanism, thereby releasing the immune system’s own capabilities to attack cancer.
Immune checkpoint therapy suppresses the activity of immune checkpoints, releases the immune brake in the tumor microenvironment, and reactivates the immune response of T cells to tumors, thereby achieving an anti-tumor effect. There are two natural ligands for PD-1, PD-L1 and PD-L2. One of the most widely studied and applied immune checkpoint inhibitors is the PD-1 / PD-L1 inhibitor. However, only about 20% to 40% of patients can actually benefit from PD-1 / PD-L1 inhibitor therapy. Two important factors are the lack of tumor-specific T cells and T Cells undergoing functional depletion.
As one of the deadliest cancers, pancreatic cancer is notoriously resistant to immune checkpoint therapies. Recently, scientists have found that the immune checkpoint VISTA is overexpressed in immune cells (especially macrophages) infiltrated into pancreatic tumors, so when PD-L1 inhibition is performed, the active VISTA pathway reduces this to a greater extent T cell immune response in this tumor. This indicates that the possible failure of using PD-1 / PD-L1 inhibitors to treat pancreatic cancer is that the active VISTA pathway still inhibits the T cell immune response, and blocking VISTA is expected to improve the efficacy of PD-L1 inhibitors on pancreatic cancer. This also means that it is possible to target other immune checkpoints to improve the efficacy of existing cancer immunotherapy.
In a new study, researchers from research institutions such as the French National Centre for Scientific Research found that as a new type of immune checkpoint inhibitor, NKG2A antibodies could potentially promote the anti-tumor effects of T cells and natural killer cells (NK cells) ability to better treat cancer patients when combined with existing cancer immunotherapy. The relevant research results were recently published in the journal Cell and the title of the paper was Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells.
The key to this research is a receptor molecule called NKG2A. The researchers found that blocking this receptor enhances the immune activity of NK cells and T cells in mice, thereby increasing the antitumor immune response. They developed an NKG2A antibody called Monalizumab. It is a humanized monoclonal antibody.
Monalizumab mAb is also safe in clinical trials. As of October 2018, the number of patients participating in the treatment reached 40, with no additional safety issues. The most common adverse reactions were fatigue, fever and headache. These data show that the combination of Monalizumab mAb and cetuximab has a higher response rate and durable response for patients.
It can be seen that as a new type of immune checkpoint inhibitor, NKG2A antibody can promote the anti-tumor immune response by enhancing the activity of T cells and NK cells, so it can be used as a complement to the first generation of cancer immunotherapy.
References:
1. Pascale André et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell, 2019, doi:10.1016/j.cell.2018.10.014.
